1. Introduction
The patient was an 11-year-old boy who presented to the Emergency Department (ED) of Children's Medical Center Hospital (CMC) with the chief complaint of shortness of breath and dry cough from the day before. The patient had no complaints of rhinorrhea or gastrointestinal symptoms.
In the past medical history, the parents originated from Afghanistan, but the patient was born and grew up in Iran. He was delivered vaginally with a body weight of 3,900 grams at 38 weeks of gestation. He had received routine newborn care without the need for hospitalization. The growth and development patterns were normal. There were no signs or evidence of eczema, allergy, or respiratory problems during infancy and early childhood. His brother and grandmother had suffered from some degree of asthma and allergic rhinitis, and his father had died three years ago following several years of working in a chemical substance plant.
At the age of three, for the first time, he experienced a prolonged cough after a cold and was treated with albuterol and beclomethasone inhalers with the diagnosis of asthma. Since then, the symptoms recurred with each cold, and they were treated with inhalers arbitrarily or as prescribed by the physicians.
On the first physical examination, he was tachypneic (RR:40). The oxygen saturation in room air was 94%. In lung auscultation, wheezing was heard in both inspiratory and expiratory phases, which were dominant in the upper zone of the thorax. He also had subcostal and suprasternal retractions. There was no evidence of nasal flaring and cyanosis. Other examinations were unremarkable.
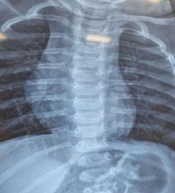
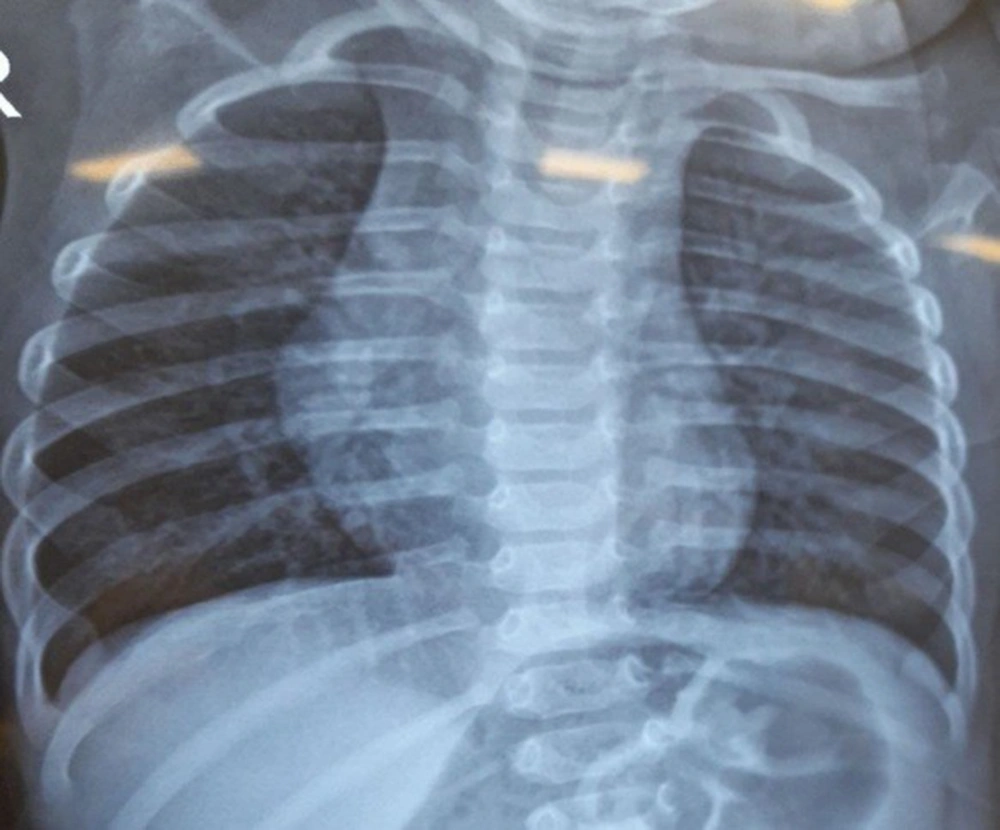
We admitted the patient to the ED. We made our patient NPO (nothing per oral) and prescribed supplemental oxygen. A chest X-ray (CXR) revealed bilateral hyperaeration (Figure 1). We requested an immunology consult; based on previous history and current symptoms, asthma attack was diagnosed. So, we prescribed a salbutamol inhaler, an Atrovent nebulizer, and 1 mg/kg body weight intravenous methylprednisolone. Initial laboratory data were normal, and arterial blood gas (ABG) was acceptable. After a few hours, the patient's general condition did not improve. We shortened the interval of the salbutamol inhaler and Atrovent nebulizer, and prescribed magnesium sulfate, which also had no effect. So, we consulted a pediatric pulmonologist for further evaluation.
2. Differential Diagnosis
Dr. Mansoureh Shariat (CMC pediatric immunologist)
The patient was an 11-old-year adolescent with a history compatible with hyperreactive airway disease who came to ED with cough and respiratory distress. The patient was treated with an initial impression of an asthma attack without improvement. So, we were faced with treatment-resistant asthma. In such situations, the following differential diagnoses should be considered and ruled out:
2.1. Allergic and Environmental Asthma
In asthma, multiple interactions between inflammatory cells, mediators, airway epithelium, smooth muscle, and the nervous system cause airway inflammation, bronchial reactivity, and reversible airway obstruction. In genetically susceptible individuals, these interactions result in asthma and manifest in attacks of breathlessness, wheezing, cough, and chest tightness following respiratory infections, air pollutants exposure, etc. (1). The patient's past and present history were compatible with an asthma attack; however, we expected a dramatic response to treatment which did not occur. In such situations, we should review the patient again and consider other differential diagnoses.
2.2. Bilateral Vocal Cord Paralysis
Vocal cord paralysis results from movement or structural disorders of the larynx, including the cricoarytenoid joint. A disorder in both the vagus nerve and the recurrent laryngeal nerve causes this condition. History is essential, as it will reveal any recent surgical procedures that may result in injury to the vagus or recurrent laryngeal nerves (2). This patient had no history of recent surgery and a predisposing medical condition for vocal cord paralysis.
2.3. Vascular Ring
The trachea and esophagus compression by vascular anomalies and branches may be symptomatic at birth or in infancy. It causes airway obstruction by compressing the trachea and may be life-threatening (3). The most common vascular ring abnormality is the double aortic arch. Respiratory symptoms include stridor in infants or young children. Therefore, it is among the differential diagnosis of upper respiratory tract infections or foreign body aspiration (4). Given that most of the vascular ring cases are present in infancy, it is less relevant for our patient.
2.4. Laryngospasm
Patients with laryngeal spasms may present symptoms such as difficulty speaking or respiratory distress. This disease is due to a temporary spasm of the vocal cords that begins acutely and unexpectedly and disappears after a few minutes. Breathing difficulty can be startling, but it is not life-threatening. Due to the short and transient nature of the laryngospasm, this condition was not consistent with the clinical course of our patient (5).
2.5. Vocal Cord Dysfunction
Vocal cord dysfunction in children is not a rare disease. The most common symptoms reported in a patient with VCD include shortness of breath, wheezing, chest pain, sore throat, or stridor, which may be common in asthma patients. Symptoms of the disease can be seen during rest or activity. Notably, the patients do not respond to conventional asthma treatment. The gold standard method for diagnosing VCD is fiber optic laryngoscopy, through which other abnormalities in the larynx can be detected (6).
2.6. Mediastinal Masses
Mediastinal masses consist of anterior, middle, and posterior masses. The most common presentations of mediastinal masses are recurrent bronchitis or pneumonia and tracheobronchial stridor due to airway compression, although it may be asymptomatic in many cases. Mediastinal tumors include neurogenic tumors and lymphomas. In lymphoma, the signs and symptoms vary depending on the location of the mass, but fever and weight loss are seen in most cases. In anterior mediastinal masses, if the tumor size is large, the patient presents with shortness of breath, which is exacerbated when lying down. In the middle mediastinum, superior vena cava syndrome has been reported following compression of blood vessels or airways. In posterior mediastinal masses, the patient is more likely to present with gastrointestinal symptoms such as dysphagia or odynophagia (7).
3. Further Evaluation
Dr. Hossein Mirlohi (CMC Pediatric Pulmonologist)
The patient was re-evaluated with the impression of a refractory asthma attack. The patient was re-examined carefully; we detected an increased inspiratory effort and biphasic wheezing, which was predominant in the inspiratory phase. We also heard inspiratory stridor somewhen.
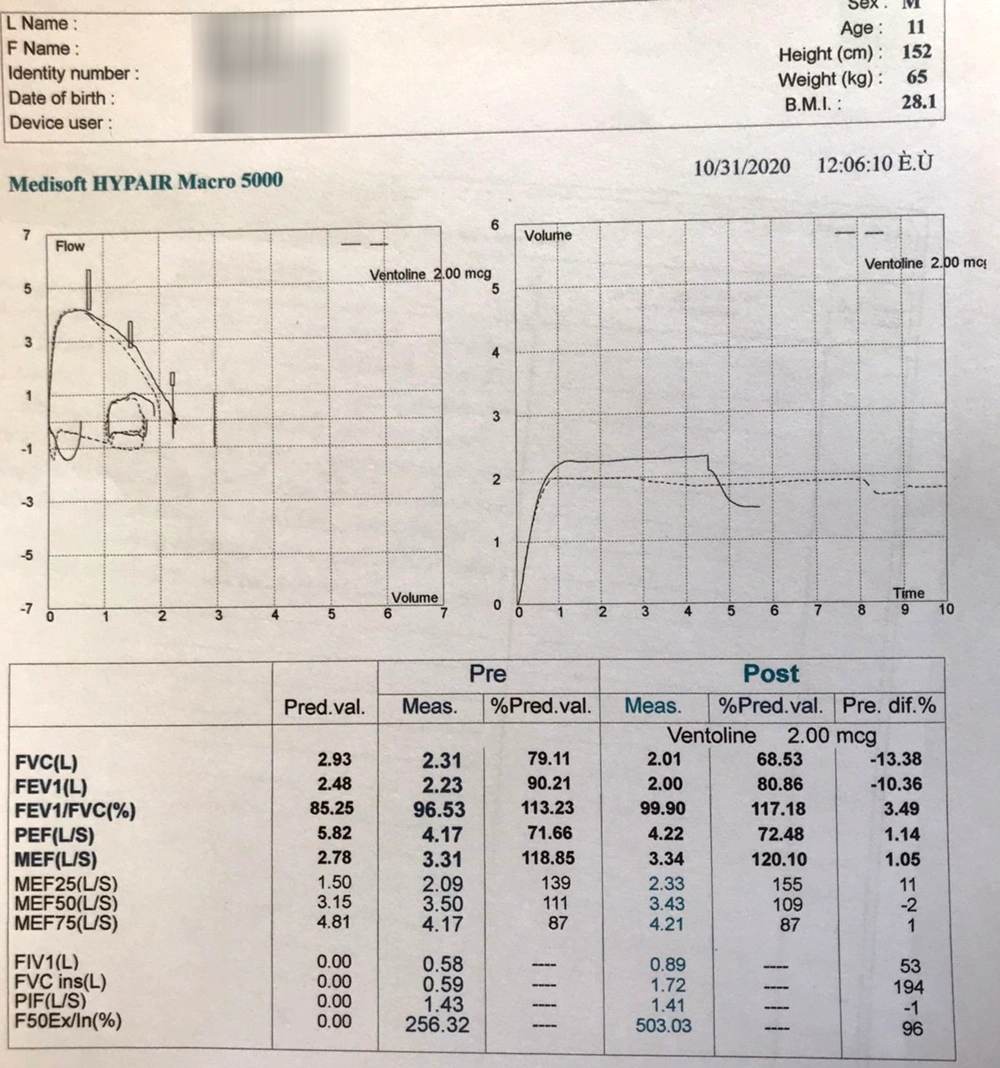
First of all, we requested a spirometry test. We found a flattening inspiratory loop in a pulmonary function test with a flow-volume loop consistent with extra-thoracic upper airway obstruction (Figure 2). Another important parameter, the force 50 expiratory inspiratory (F50Ex/I), was 256%, which normally should be less than 90%, indicating upper respiratory tract obstruction.
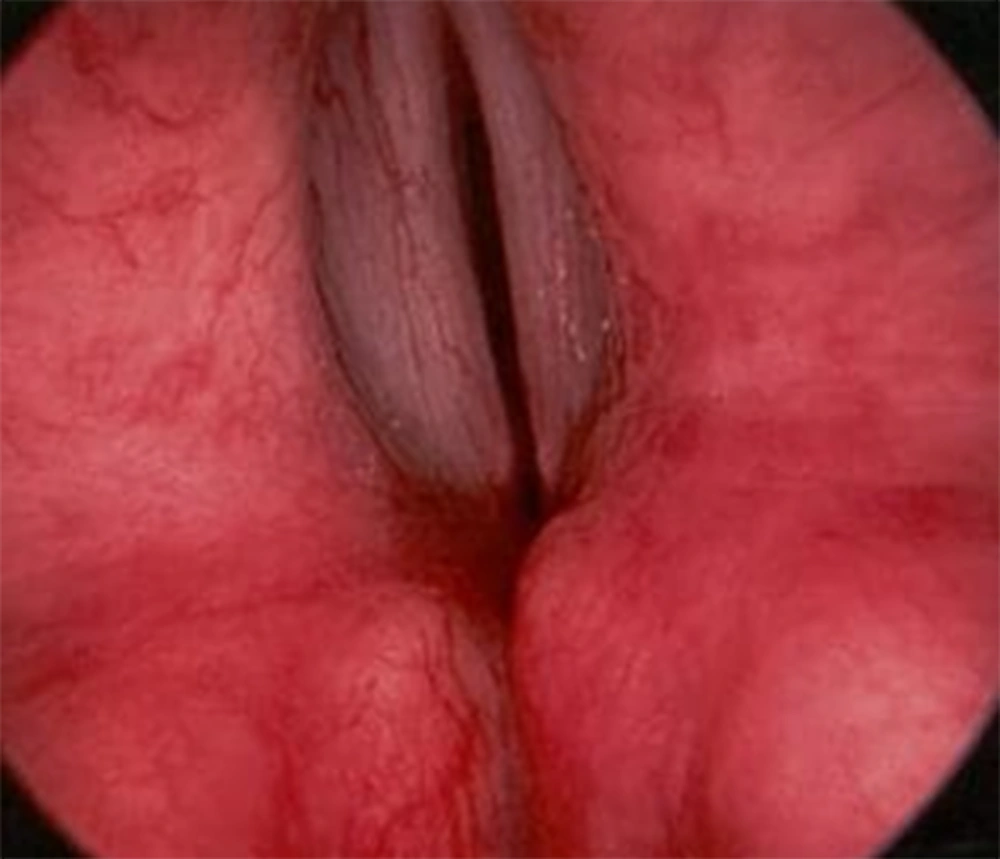
According to the evidence in the physical examination (presence of stridor and inspiratory wheezing) and spirometry findings, the patient underwent fiberoptic bronchoscopy to rule out anatomical abnormalities. Bronchoscopy revealed vocal cord closing during the inspiratory phase (Figure 3) in favor of vocal cord dysfunction (VCD). We also detected inward collapse of flaccid supraglottic structures during inspiration, indicating the presence of adult-onset laryngomalacia.
4. Clinical Diagnosis
VCD and Adult-type Laryngomalacia
5. Discussion of Management
In VCD, there is abnormal adduction of the vocal cords in inspiration. In other words, the movement of the vocal cords is seen inconsistently during a respiratory cycle, which obstructs the airways. The most important differential diagnosis of VCD is asthma, in which the patient is repeatedly treated with medications such as inhaled or systemic corticosteroids and bronchodilators but does not respond to treatment (8, 9).
As mentioned earlier, this patient was frequently treated for asthma. Considering the results of spirometry and abnormal fiberoptic bronchoscopy findings, we also determined adult-onset laryngomalacia in this patient. This condition could explain the overlap of asthma and vocal cord symptoms. Adult-onset laryngomalacia is marked by severe dyspnea, stridor, and mild wheezing unresponsive to beta-agonists and other asthma medications. Flexible fiberoptic laryngoscopy reveals a thickened posterior arytenoid mucosa that readily prolapses into the glottis with inspiration (10). No universal definition and diagnostic criteria are determined for VCD, and risk factors are based on cohort studies. As a result, we do not accurately know the incidence of the disease (11)
Vocal cord dysfunction should be considered in patients with refractory asthma or chronic cough. A patient with VCD has symptoms such as shortness of breath, wheezing, coughing after exercise, or movement of respiratory symptoms despite a stimulus. In VCD, shortness of breath starts suddenly, usually lasts more than two minutes, and resolves independently. These symptoms occur in inspiration. It is localized in the throat or the upper part of the trachea (12). In patients with VCD, chest stiffness is seen for unknown reasons but is less than in patients with asthma. Patients with VCD are initially treated with prednisone after being diagnosed with asthma, and this treatment may be repeated several times. In some cases, they respond to treatment.
In a retrospective report, Newman et al. reported that the average prednisone dose in patients with VCD was 29.2 mg daily. They also had 9.7 emergency visits and 5.9 admissions per year. Notably, 28% of these patients needed respiratory support by intubation and mechanical ventilation, which is relatively high. In some patients, biphasic wheezing was reported (13). It is essential to mention that when VCD is diagnosed, it does not necessarily mean that the asthma diagnosis is incorrect. Indeed, VCD and asthma may be present simultaneously. Based on references, 20% of patients with VCD may also have asthma simultaneously (14).
Medical and psychological underlying disorders should be ruled out in a patient diagnosed with VCD. Questions should be directed at detecting the possible contributing factors. In addition, during the physical examination, physicians should look for evidence of post-nasal drip, Gastrointestinal Reflux (GER), and Laryngopharyngeal Reflux (LPR). Clinical signs of GER and post-nasal drip include chronic cough, headache, sore throat, allergy, increased coughing in the supine position, nausea, nasal congestion, and worsening cough at night (14). Our patient had no problem in this regard.
Diagnosing patients with potential primary functional disorders is essential, so we should evaluate them psychologically by taking a history of mental illness in the patient or those around him/her and asking about substance abuse. Furthermore, several patients may experience anxiety and/or depression due to being stigmatized as having a serious and even potentially life-threatening condition (e.g., asthma) that seems to be getting worse despite aggressive therapy.
The pathophysiology of VCD is very complex. Therefore, managing the disease requires the simultaneous cooperation of several disciplines, including psychiatry and speech therapy (15). Speech and language pathologists/therapists or respiratory therapists treat patients through several methods: First, they teach patients cough control techniques and throat-clearing suppression and then teach them how to control their laryngeal response in the face of stimuli and overcome an acute attack. Other specialties that help manage the disease include pulmonologists, general pediatricians, internists, otolaryngologists, allergists, occupational medicine specialists, psychiatrists, psychosocial medicine, rehabilitation medicine, and vocational counselors.
In an acute attack, the use of Heliox (80% helium/20% oxygen) or simple continuous positive pressure of the airway with intermittent positive pressure is effective (16). Topical lidocaine is effective in patients with acute symptoms. Superior laryngeal blocks with clostridium botulinum toxin have been used in some severe cases (17).
6. Follow-up
The patient's general and respiratory conditions improved. We discharged the patient and referred him to the center's pediatric psychiatric clinic and speech therapist. The psychiatrist took a careful and detailed history, revealing several psychiatric and psychological abnormalities. There were likely in the context of several crises the patient had experienced, including father loss in childhood and refugee problems. He experienced some pseudo seizures later in the course of the disease, resulting in hospital admission once. Medications were started for him. He responded to the treatment well, and the cough and dyspnea attacks resolved gradually. There was no need to prescribe any other medication on follow-up visits to the pulmonology clinic.
7. Discussion of Psychiatric Issues
Dr. Mohammad Effatpanah (CMC Pediatric Psychiatric)
The patient was referred to our clinic. According to a psychological assessment of the Minnesota Multiphasic Personality Inventory (MMPI) and Structured Clinical Interview for DSM-5 Axis II Disorder (SCID-II) and also a child & adolescent psychiatrist interview, the patient had somatic symptoms disorders (functional speech and voice disorders) and mild to moderate depressive disorder. Misono et al. reported a high prevalence of life distress in patients presenting with voice concerns (18).
Watson et al. noted that these patients are prone to different mental health problems, such as difficulty in peer relationships. They also described several stressors for this disorder, including relationship problems, parental discordance, death of a loved one, etc. (19). In this case, he was an immigrant and recently lost his father; he also lived in a chaotic family. Jordan et al. discussed the high prevalence of psychiatric problems such as anxiety, depression, and somatic concerns in these patients (20).
In practice, the physician should be aware of different types of functional (or psychogenic) speech and voice disorders. Several red flags are described for functional speech and voice disorders to distinguish them from organic motor speech disorders, such as suggestibility, distractibility, acute onset, variable severity during different speech examination activities, paradoxically increased muscle contraction with fatigue, the potential for rapid reversibility or improvement of symptoms and denial or indifference to abnormal speech/voice (21).
Guglani et al. described different methods for the treatment of VCD; the studies were small case series or individual case reports using interventions such as psychotherapy, behavioral therapy, use of anti-anxiety and anti-depressant medications, and hypnotherapy in conjunction with breathing exercises taught by speech therapists for symptomatic relief of these disorders. Anti-depressant, in general, can improve symptoms of these disorders in combination with other behavioral/psychological interventions (22).
8. Final Diagnosis
Vocal cord dysfunction due to several psychiatric problems and adult-type laryngomalacia
9. Conclusions
Vocal cord dysfunction may mimic asthma; early diagnosis may avoid unnecessary hospital admission and asthma medication consumption without indication. Management of VCD requires recognition and treatment of underlying medical and psychological disorders and referral to a trained speech or respiratory therapist.
10. Take-home Messages
Patients with refractory asthma should be evaluated for underlying diseases.
Patients with VCD should be assessed for underlying medical and psychological disorders.