1. Introduction
According to the World Health Organization, on April 15, 2022, severe acute hepatitis of unknown origin was reported in children under 16 years in the United Kingdom and Northern Ireland (1, 2). Then, similar cases were reported in other countries that are rising (2). The number of cases has reached more than 169 so far, and cases have been reported from at least 11 European countries and one country in America (2).
Clinical syndrome in these cases is diagnosed as severe acute hepatitis with a marked increase in transaminases, often with jaundice and sometimes with gastrointestinal symptoms such as vomiting as a prominent feature in children up to 16 years of age. Sometimes, it requires transfer to specialized pediatric liver units, and several children have undergone liver transplantation (3, 4). According to current information, the patients did not have a history of international travel or contact with other countries (3). The leading cause of acute hepatitis is currently unknown, and in laboratory tests, hepatitis A, B, C, D, and E were ruled out (1). Some patients have been identified with Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2) or adenovirus (4). Although the possible role of adenovirus or SARS-CoV-2 has been suggested as a hypothesis, further studies on other infectious and non-infectious agents are needed to assess and manage the risk.
In this regard, we reviewed and presented three cases of severe acute hepatitis of unknown origin, and the measures taken in these children referred to a children's hospital medical center in Tehran, Iran.
2. Case Presentation
2.1. Case 1
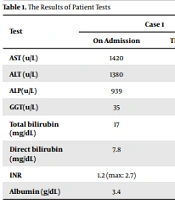
A 12-year-old boy with a history of COVID-19 infection 50 days ago was admitted with jaundice on March 12, 2022. All laboratory tests, including liver enzymes, blood cells, ceruloplasmin, autoantibodies related to autoimmune hepatitis, and SARS-CoV2, were performed according to Table 1. Hepatitis B surface antigen (HBsAg), hepatitis C virus (HCV) antibodies (Ab), hepatitis A virus (HAV) IgM, HEV IgM, Epstein-Barr virus (EBV) IgM, anti–liver-kidney microsomal Ab (LKM Ab), anti-smooth muscle antibody (ASMA), antinuclear antibodies (ANA), and total IgG were all negative. However, SARS-CoV-2 IgG was > 50, and SARS-CoV-2 IgM was negative. Also, the patient's history did not indicate using specific herbal medicines or hepatotoxic drugs.
Multisystem Inflammatory Syndrome of Children (MISC) markers were also negative, and normal echocardiography and chest radiographs were observed. In addition, International Normalized Ratio (INR) gradually increased to 2.7 during hospitalization. While performing transplant preparation measures, 1 mg/kg/day intravenous methylprednisolone was started for the patient, which was gradually reduced by liver tests including INR and bilirubin, and after 10 days, the patient was discharged with oral prednisolone. One week later, the liver function tests decreased significantly at the outpatient visit, and the prednisolone dose was gradually tapered.
| Test | Case 1 | Case 2 | Case 3 | |||
|---|---|---|---|---|---|---|
| On Admission | The Last Tests | On Admission | The Last Tests | On Admission | The Last Tests | |
| AST (u/L) | 1420 | 82 | 970 | 73 | 1033 | The same |
| ALT (u/L) | 1380 | 126 | 1270 | 200 | 2080 | The same |
| ALP(u/L) | 939 | 650 | 399 | 400 | 913 | The same |
| GGT(u/L) | 35 | 88 | 251 | 251 | 100 | The same |
| Total bilirubin (mg/dL) | 17 | 2.3 | 41 | 7 | 8.2 | 6 |
| Direct bilirubin (mg/dL) | 7.8 | 1.2 | 21 | 4.2 | 4.9 | 3.9 |
| INR | 1.2 (max: 2.7) | 1.2 | 2.2 | 1.1 | 1.1 | 1.1 |
| Albumin (g/dL) | 3.4 | 3.5 | 3.5 | 3.5 | 4.9 | 4.3 |
2.2. Case 2
The second case of a six-year-old girl with jaundice from a week ago was admitted on April 11, 2022. Examinations showed no evidence of chronic liver disease. However, the liver was enlarged, and jaundice was observed.
Like the first case, she underwent liver diagnostic tests, including liver enzymes, biochemistry, and a variety of viral hepatitis (Table 1). Normal C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were reported. Also, HCV Ab, HBs Ag, EBV IgM, CMV Ab, HAV IgM, HEV IgM, LKM, ASMA, ANA, SARS-CoV-2 IgM, and IgG, and total IgG were reported negative. In addition, the patient's history did not indicate the use of specific herbal medicines or drugs.
Due to the progressive course of liver dysfunction, especially raising INR and bilirubin and the impossibility of liver transplantation, this child started methylprednisolone at a dose of 1 mg/kg/day. She was discharged after one week with a reduction of INR and other liver tests. After discharge, the dose was gradually reduced. The latest results of patient tests on May 7, 2022, are shown in Table 1.
2.3. Case 3
The third case is a 12-year-old boy with symptoms of jaundice hospitalized on May 8, 2022. There were no signs of chronic disease. Similar to the first and second cases, functional and diagnostic tests were performed according to Table 1. Normal serum ceruloplasmin was reported. Besides, HCV Ab, HBs Ag, EBV IgM, CMV Ab, HAV IgM, HEV IgM, LKM, ASMA, ANA, and COVID-19 and adenovirus polymerase chain reaction (PCR) were negative. Also, the drug history was negative for any hepatotoxic agent. He is currently undergoing conservative treatment.
3. Discussion
As mentioned in the introduction, more than 300 cases of severe acute hepatitis of unknown origin have been reported worldwide in children under 16 years of age (3). These patients often have highly elevated transaminases, severe jaundice, and sometimes gastrointestinal symptoms. Some cases have undergone liver transplantation due to acute liver failure.
In our center, as a nationwide referral center, the number of hospitalizations for acute hepatitis has increased since early March 2022 compared to the same period last year and previous months. The three patients presented were in the age range of 6 to 13 years, and the etiological studies, including Wilson's disease, drug-induced hepatitis, contact with toxins, and infectious hepatitis such as hepatitis A, B, C, D, E, EBV, CMV, and autoimmune hepatitis, were all negative. One of the three patients had a very high SARS-CoV-2 IgG, but there was no evidence of active lung infection or MISC and fever. However, a PCR adenovirus examination was performed on the third case, and the result was negative. Therefore, it is impossible to comment on the association between COVID-19 and severe acute hepatitis of unknown origin in these children.
In two of the three patients, due to the progressive fulminant hepatic failure, coagulopathy, jaundice, and the absence of fever and other specific evidence of infection, intravenous methylprednisolone was started at a rate of 1 mg/kg/day (5). Within 48 hours of starting the drug, the worsening course of fulminant liver failure stopped, and liver function tests improved gradually. Thus, they were excluded from the phase of acute liver failure and were discharged with oral prednisolone. In outpatient visits, the prednisolone dose gradually decreased, and liver tests almost reached the near-normal range. Given these cases, using corticosteroids in children with severe acute hepatitis of unknown origin may be helpful, given the potential for immune-mediated reactions (5).
3.1. Conclusion
In Iran, like worldwide, severe acute hepatitis of unknown origin is increasingly occurring in children. General practitioners and pediatricians are advised to examine the underlying causes and consider severe acute hepatitis of unknown origin when visiting children under 16 years of age with severe jaundice and very high transaminases. Due to the uncertain course of the disease and diagnostic ambiguities, it is better, if possible, for patients to be hospitalized and monitored for liver function. However, the efficacy and safety of corticosteroids in these patients cannot be conclusively commented on, but in two of the three patients presented, starting corticosteroids halted the progression of acute liver failure, and the patients recovered.