1. Background
Congenital heart disease (CHD) is the most common structural congenital disease in children. The disorder is seen in six to 13 per 1,000 live births. It varies in complexity and sometimes does not affect a person’s well-being or even remains unrecognized for years. However, one-third of these patients develop a severe disease that should be treated in infancy. Today, with advances in surgical and non-surgical interventions, life expectancy has reached about 80% in 20 years (1, 2).
The most common CHDs are ventricular septal defect, atrial septal defect (ASD), patent ductus arteriosus (PDA), and tetralogy of fallot (TOF) (3, 4). In the past three decades, interventional methods have improved in treating these diseases, replacing surgery and decreasing anesthesia and bypass time (3). However, children treated are prone to developmental disorders due to prenatal complications such as hypoxia and complications from therapeutic modalities such as decreased blood supply to the brain or stroke (4). Children with CHD also show decreased executive functions and behavioral problems (5-7). Executive functions refer to the individuals’ ability to plan their behaviors to achieve a specific goal, such as identifying problems, providing solutions, choosing the best solution, and regularly monitoring and correcting performance (8).
Most researchers consider the evolution of executive functions as one of the most important achievements of preschool children and regard it as a criterion for predicting successful school performance and adjustment (5). Self-control requires acquiring cognitive skills that lead to problem-solving and behavioral adaptation in new situations. These skills are generally called executive functions. Executive function helps review and monitor actions to perform a task correctly despite multiple triggers (6, 7). Children with executive dysfunction have difficulty achieving educational goals that are not explained by a learning disorder. These children cannot apply their knowledge in everyday life (9). Due to a decline in executive function and behavioral problems at school, CHD patients need to be screened and rehabilitated.
2. Objectives
This study aimed to compare executive function and behavioral and emotional problems between successfully surgically and interventionally treated children with CHD without any reported complications and a normal group.
3. Methods
This retrospective cross-sectional study enrolled children aged eight to 16 years with CHD treated with surgery or catheterization. An age-matched control group was also recruited.
3.1. Sample Size and Patient Selection
Sixty patients with a history of cardiac problems (30 in the surgical group and 30 in the interventional group) and 30 normal subjects were enrolled who were treated in a hospital affiliated with Shiraz University of Medical Sciences. The samples were randomly selected from the pediatric cardiology and cardiac surgery data bank. Patients with complicated surgery or intervention courses, neurological problems, significant motor problems, developmental disabilities, psychiatric problems, learning disorders, or hyperactivity were excluded. All subjects were studying in ordinary schools and had reading and writing abilities.
3.2. Tools
The researcher called all patients and invited them for face-to-face interviews. According to the proposed research protocol, each subject was invited with one parent to the research. The Strengths and Difficulties Questionnaire (SDQ), the parent version, was completed by the companion parent. The Stroop test, Trail Making Test (TMT), cancellation test, and auditory and visual memory span test were done for children.
3.3. Evaluation of Executive Function
The executive function is divided into working memory (visual and verbal), cognitive flexibility (the ability of a person to shift attention from one task to another), and inhibitory control (the ability not to perform a dominant response but select the appropriate solution to do the job correctly) (10). The following tests were used to evaluate these executive functions.
3.4. Stroop Test
This test is widely used for selective or focused attention and response inhibition. It is a laboratory model for measuring selective attention (11). We used this test as a set-shifting index.
3.5. Trail Making Test
The TMT assesses cognitive functioning areas such as processing speed, sequencing, intellectual flexibility, and visual or motor skills (12). In the present study, the subjects were in their childhood and adolescence. The graphic form of the test was used instead of the digit form. The participant was supposed to count the points of each circle rather than the numbers. The time required to complete the tests was recorded. This test is used to evaluate simple and complex selective attention.
3.6. Cancellation Test
It was used as a paper-pencil test to assess visual scanning ability. This study used a cancellation test to evaluate the error of deletion (omission) and commission and the test run time. Therefore, sustained attention capacity, visual search accuracy, and response activation and retention were estimated (13). This test was used as a sustained attention index in our study.
3.7. Auditory Digit Span Test
The two parts of the test, forward and backward (reverse), are separately applicable. This test was used to evaluate the auditory memory span. In the backward digit test, the subject reads a series of similar numbers and is asked to repeat them in reverse. The reverse digit test was done even if the subject was weak in the forward digit test. This test is used to evaluate simple working memory (13). In the third step, the child had to write only the numbers read by the female speaker, and the number of correct rows was compared. This test was used as a hard-working memory index.
3.8. Visual Memory Span Test
Several patterns, from simple to complex, are presented to the subject for four seconds. Then, the subject is asked to imitate the pattern observed in the form. In this test, the number of correctly painted patterns was compared (14).
3.9. Parent Strengths and Difficulties Questionnaire
The SDQ is a short screening tool increasingly used to identify children and adolescent’s behavioral and emotional problems. It assesses five main subtypes of psychiatric symptoms: conduct problems, functionality, emotional symptoms, peer problems, and socially desirable behavior. The first four subtypes give the overall score of the problems. The SDQ has an affective score indicating that the severity of a child’s problems is large enough to interfere with his/her daily life (15). Scores obtained from parent and teacher versions of SDQ correlate positively. Comparing performance indices and the overall score of psychiatric diagnostic problems also showed that SDQ is well-validated (16). The Persian version of the SDQ has good psychometric properties and can be a helpful screening tool for Iranian children with behavioral and emotional problems (17).
The Shiraz University of Medical Sciences Ethics Committee approved this work (IR.sums.med.rec.1396.5251). All parents of the study participants completed informed consent forms.
3.10. Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) version 22 software was used in this study. Descriptive data were reported as mean ± standard deviation (SD). Data with normal distribution were statistically analyzed using the t test, and data with abnormal distribution were analyzed using the Mann-Whitney U test. Pearson correlation was used to investigate the statistical relationship between the variables. A P value of less than 0.05 was statistically significant.
4. Results
The mean age of the subjects was 11.58 ± 2.45 years. The sample included 51.7% girls and 48.3% boys. All subjects began primary school education and were right-handed.
The atrial septal defect was the most common disease in the interventional group, and the single ventricle with total cavopulmonary connection was the most common disease in the surgical group.
In the interventional group, 16 ASD closures, five VSD closures, four ductus closures, three balloon angioplasties of coarctation, one balloon valvuloplasty of aortic stenosis, and one pulmonary balloon valvuloplasty were enrolled.
In the surgical group, there were 11 patients with total cavopulmonary connection, eight with repaired TOF, three with ASD closures, three with VSD closures, two with Rastelli operations, one with complete atrioventricular septal defect closure, one with transposition of the great artery, and one with hypertrophic cardiomyopathy with myectomy of the left ventricular outflow tract.
4.1. Behavioral Problems Analysis
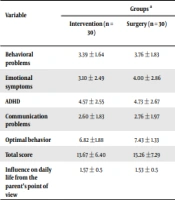
There was no significant difference between the two groups of patients in behavioral subscales. However, the effect of behavioral problems on the child’s life was significantly higher in the surgical group (Table 1).
| Variables | Groups a | P Value | ||||
|---|---|---|---|---|---|---|
| Intervention (n = 30) | Surgery (n = 30) | Normal (n = 30) | Between Surgery and Normal | Between Intervention and Normal | Between Surgery and Intervention | |
| Behavioral problems | 3.39 ± 1.64 | 3.76 ± 1.83 | 1.76 ± 1.75 | 0.0001 | 0.0001 | 0.269 |
| Emotional symptoms | 3.10 ± 2.49 | 4.00 ± 2.86 | 2.25 ± 1.97 | 0.002 | 0.002 | 0.203 |
| ADHD | 4.57 ± 2.55 | 4.73 ± 2.67 | 3.99 ± 2.29 | 0.139 | 0.139 | 0.418 |
| Communication problems | 2.60 ± 1.83 | 2.76 ± 1.97 | 1.67 ± 2.04 | 0.005 | 0.005 | 0.667 |
| Optimal behavior | 6.82 ±1.88 | 7.43 ± 1.33 | 8.11 ± 1.75 | 0.009 | 0.009 | 0.216 |
| Total score | 13.67 ± 6.40 | 15.26 ±7.29 | 10.05 ± 5.76 | 0.0001 | 0.0001 | 0.224 |
| Influence on daily life from the parent’s point of view | 1.57 ± 0.5 | 1.53 ± 0.5 | 0.12 ±1.34 | 0.0001 | 0.0001 | 0.0001 |
Abbreviation: ADHD, attention deficit hyperactivity disorder.
a Values are expressed as mean ± standard deviation.
Both patient groups showed higher scores in emotional symptoms, behavioral problems, and communication problems and lower scores in optimal social behavior than in the normal group, but there was no difference in overactivity symptoms (Table 1).
The child’s daily life was examined from the parent’s point of view. Forty-five percent of parents believed in the influence on one of the areas of family, friendship, school, or leisure activities, and 55% did not believe in this effect (Table 1).
The set-shifting, working memory, visual memory, simple selective attention, and complex selective attention analysis showed no significant difference between the two groups of patients. However, there was a statistically significant difference from the normal group (Table 2).
| Variables | Groups a | P Value | ||||
|---|---|---|---|---|---|---|
| Surgery (n = 30) | Intervention (n = 30) | Normal (n = 30) | Between Surgery and Normal | Between Intervention and Normal | Between Surgery and Intervention | |
| Set shifting | -4.44 ± 5.16 | -5.92 ± 4.89 | 0.01 ± 1.05 | 0.0001 | 0.0001 | 0.272 |
| Memory span | 2.95 ± 1.27 | 3.46 ± 1.22 | 5.04 ± 1.31 | 0.0001 | 0.0001 | 0.037 |
| Working memory | 1.73 ± 1.27 | 2.17 ± 1.73 | 5.11 ± 0.35 | 0.0001 | 0.0001 | 0.181 |
| Hard work memory | 1.85 ± 1.21 | 2.02 ± 0.85 | 5.07 ± 0.70 | 0.0001 | 0.0001 | 0.604 |
| Visual memory | 2.25 ± 0.90 | 2.35 ± 1.11 | 5.10 ± 1.41 | 0.0001 | 0.0001 | 0.722 |
| Simple selection attention | 131.12 ± 64.94 | 108 ± 52.85 | 22.93 ± 12.28 | 0.0001 | 0.0001 | 0.163 |
| Complex Selective attention | 91.00 ± 38.15 | 122.36 ± 103.23 | 48.97 ± 28.57 | 0.0001 | 0.038 | 0.377 |
a Values are expressed as mean ± standard deviation.
Patients in the surgical group had significantly less memory span than the interventional group. Also, both groups of patients were significantly lower than the normal group in memory span (Table 2).
In the sustained attention test, there was a significant difference between the two groups of patients in the correct selection (correct), omit removal, and omit error. However, there was no difference in the duration of the test. In this area, the performance of the normal group was not measured (Table 3).
| Surgery | Intervention | P-Value | |
|---|---|---|---|
| Correct | 14.8 ± 4.88 | 17.23 ± 2.06 | 0.0001 |
| Omit | 3.68 ± 4.08 | 1.73 ± 2.06 | 0.020 |
| Commit | 0.55 ± 2.40 | 0.03 ± 4.08 | 0.021 |
| Time | 111.73 ± 42.71 | 113.16 ± 49.25 | 0.628 |
5. Discussion
Significant advances have been made in treating CHD, either surgically or non-surgically, leading to decreased mortality and increased life expectancy in CHD children (1, 2). In this regard, more neurodevelopmental disorders are detected in both surgically and interventionally treated groups, and the most important is the low level of cognitive skills (8). These patients appear to have more impaired memory and task organization. These children also have behavioral disorders in hyperactivity, impulsivity, conduct, antisocial behavior, isolation, depression, and anxiety (9).
Medical treatment is to increase not only life expectancy but also life quality. Executive dysfunction significantly predicts academic achievement, social communication, employment, and treatment adherence (16).
Some studies focused on intellectual quotient (IQ). Ryberg et al. examined two groups of congenital heart patients who underwent surgery and catheterization regarding IQ (8). Totally 228 children were classified according to age and disease severity. The results showed that 83% of children with CHD had expected or even higher than normal IQ (8). Low IQ was associated with the family’s economic level and heart disease severity. Although intelligence is one of the predictors of a child’s performance at school, executive function also should be considered (8).
Wray and Sensky compared 45 patients with CHD who underwent heart surgery with 51 patients who underwent bone marrow transplantation and 51 normal individuals (18). The IQ was generally in the normal range in patients with heart disorders, but they had lower scores in data analysis and processing speed (18). Chronic disease can be one of the causes of poor performance, but patients with congenital heart defects were lower in information processing and academic achievement than those with other chronic diseases (18).
Our study also considered executive function. Also, according to the parent’s point of view, children included in this study had a normal IQ without particular educational needs. The surgically treated patients had lower performance in memory span and sustained attention than the interventional group. The severity of CHD can explain this difference. The surgical group had more complex CHD (single ventricle, TOF, and aortic coarctation) than the catheterization group (ASD, VSD, and PDA).
In some studies, patients with complex CHD were impaired in various areas, such as memory, academic status, visual-spatial perception, executive function, and attention (10, 19, 20). In complex CHD patients, more brain abnormalities have been found in magnetic resonance imaging (MRI), although most of these disorders appear to be acquired, and there is no significant relationship between abnormal findings in MRI and neurodevelopmental tests (21). Impairment of information processing speed, reaction rate, attention, selective attention, fine movements, working memory, and spatial-visual skills is more common in patients with complex heart abnormalities than in patients with simpler abnormalities (22). Klouda et al. showed that patients with more severe congenital heart defects had more surgeries associated with more executive dysfunction. The risk of acquired brain injuries during surgery, such as strokes, should also be considered (19).
On the other hand, overprotection, inactivity, parental dependency, and high parental anxiety in patients undergoing surgery, especially in cyanotic patients, can lead to a lack of cognitive skills (8). Patients with severe CHD appear to have a greater risk for congenital brain abnormalities (18), which are also related to physiological events due to fetal and chromosomal abnormalities (23, 24). Also, these patients are exposed to acquired executive dysfunctions due to multiple surgeries, hypoxia during and after surgery, seizures, and physical inactivity (25-27). Complex congenital heart defects can predict neurodevelopmental disorders in adults, although children with simple congenital anomalies such as ASD have also defects compared to the normal population (28).
In our study, both groups of patients had lower performance in attention, set-shifting, memory span, working memory, and visual memory. Some research focused on surgical factors explaining developmental neurological disorders in patients with congenital heart abnormalities (28, 29). Prolonged deep hypothermic circulatory arrest and extracorporeal membrane oxygenation are the risk factors for neurodevelopmental disorders (28). Interestingly, complications during surgery could only justify 5 - 8% of developmental disorders (14, 30). Despite the advances in surgical procedures, there is no reduction in neurodevelopmental disorders. The nature of the disease, preoperative factors, and factors during surgery are responsible for neurodevelopmental disorders in treated CHD patients, and surgical treatment does not seem to play a role in improving executive function (18).
The present study results also confirmed that patients with CHD, regardless of intervention type or disease severity, had executive function lower than the average. However, in a meta-analysis by Karsdorp et al., the executive function in patients with simple heart disease was not significantly different from normal (31).
In this study, the SDQ, the parent version, was used to assess emotional symptoms, behavioral problems, communication problems, hyperactivity, and desirable behavior and the impact of behavioral disorders on a patient’s life (17). The family report of the effect of behavioral disorders on patient life was significantly higher in the surgical group than in the non-surgical group (P < 0.0001).
The patient groups had higher scores in emotional symptoms, behavioral problems, and communicational problems and lower scores in desirable behavior than the control group There was no difference in the area of hyperactivity score in interventionally and surgically treated group. Similar research suggests that people with chronic illnesses are more prone to behavioral disorders (32-35). Also, behavioral and emotional disorders are higher in patients with congenital heart defects than in the normal population, and these disorders are not related to disease severity (16, 21). Kramer et al. compared 128 patients with CHD with 89 normal people in the control group (36). They found that children with heart disease had more behavioral problems, feelings of inferiority, and anxiety (36).
In a randomized controlled trial, Bellinger et al. compared 155 children aged four to eight who underwent arterial switch operations with a control group (33). The scores for behavioral disorders, children’s behavioral checklists, and teachers’ checklists were higher in the patient group than in the normal group. This study suggested that patients with CHD are at risk for behavioral disorders (33). Our study showed more behavioral problems in CHD patients than in normal children, except for attention-deficit hyperactivity disorder, because these patients were excluded.
The analysis of the impact of the above behavioral problems on the child’s daily life from the parent’s point of view showed that 45% of the parents believe that they have been affected by one of the family domains, school friendships or recreational activities, and 55% of the parents did not believe in this. The effect was unbelievably much lower than expected. This issue can be due to more parents’ attention to physical illness than behavioral problems, the level of tolerance of the family to the sick child, family refusal to express behavioral disorders due to cultural issues, and the participants’ age. Some studies, such as a meta-analysis in Norway, which looked at the executive and psychological functioning in children and adolescents with CHD, have shown that psycho-behavioral disorders appear in older children, and they show internal disorders such as depression and anxiety more than external disorders such as hyperactivity and behavioral problems (31).
5.1. Study Limitations
The small sample size, the high variability of CHD, and the lack of information before the operation were the limitations of this study. There were dissimilarities between intervention and surgery groups in this study. The intervention group consisted of simple noncyanotic cases, while the surgery group contained cyanotic and complex diseases.
5.2. Conclusions
Interventionally treated patients with CHD had better performance than surgically treated patients, and the trend to treat these patients non-surgically can improve these patients’ executive function. However, patients with congenital heart malformations, regardless of disease severity or treatment, had poor performance compared to normal subjects and suffered from behavioral disorders affecting their daily lives. Therefore, it is necessary to include diagnostic and therapeutic interventions for executive function and behavioral problems in the treatment protocol for these patients. Family education can help faster diagnosis. Disorders of cognitive flexibility can also predict behavioral problems.