1. Background
The ventricular septal defect (VSD) has been identified as the most frequent form of congenital heart disease. With the advent of echocardiography and the increasing experience of operators, VSD has been diagnosed in 5 per 1000 live births (1, 2). Ventricular septal defect is categorized into perimembranous, muscular, outlet, and inlet types, and transcatheter VSD closure is typically used for muscular and perimembranous types. This method has been connected with several complications, including residual shunting, device embolization, hemolysis, aortic and tricuspid regurgitation, and complete heart block.
In general, transcatheter device closure of perimembranous ventricular septal defect (Pm VSD) remains challenging, and there is little data on long-term results. Since the defect is close to the aortic tricuspid valves and the conduction system, there is a possibility of the device’s influence on the aortic and tricuspid valve function (3).
The immediate and short-term results of transcatheter VSD closure with the Amplatzer VSD Occluder have been well documented (4, 5). However, there are limited long-term follow-ups to assess arrhythmia and other complications of percutaneous closure of VSDs.
2. Objectives
The present study described the long-term follow-up results of transcatheter closure of perimembranous and muscular VSDs using different VSD devices in 409 selected patients at our medical center. Also, we compared a residual shunt in patients with single-hole and multiple-hole VSDs.
3. Methods
This is a retrospective study of all diagnosed patients with perimembranous or muscular VSD who underwent percutaneous device closure from March 2009 to February 2020 in a tertiary cardiovascular medical and research center. The study protocol was approved by the medical ethics committee.
The study aimed to evaluate complications of transcatheter VSD closure and compare the results of percutaneous VSD closure in patients with single-hole and multiple-hole VSDs. Also, possible risk factors and complications during long-term follow-up after transcatheter VSD closure were evaluated. For a better assessment, various types of devices and delivery systems were used for VSD closure.
All patients were evaluated using transthoracic echocardiography (TTE) (GE Vivid3 machine), including M-mode, two-dimensional, and color Doppler examinations. The size and types of VSDs were measured by a standard 4-chamber view, a 5-chamber view, and a parasternal long and short axis view.
A standard 12-lead electrocardiogram (ECG) was done in all patients. The patients’ general characteristics are reported in Table 1.
| Variables | Values |
|---|---|
| Patients (n) | 409 |
| Gender | |
| Male | 209 (51) |
| Female | 200 (49) |
| Age (y) | 7 ± 1 (2 - 15) |
| Weight (kg) | 27.3 (10 - 75) |
| BMI | 20.1 (17 - 25) |
| Hb | 12.2 (10.5 - 14) |
| Heart rhythm | Normal sinus rhythm |
| NYHA functional class | 1,2 |
| Ventricular septal defect size (mm) | 4.8 ± 2 (2 - 15) |
| Ventricular septal defect type | |
| Perimembranous | 346 (84) |
| Muscular | 63 (16) |
| QP/QS | 1.5 ± 0.5 (1.4 - 4.5) |
| Single hole ventricular septal defects | 259 (63.4) |
| Multiple hole ventricular septal defects | 150 (36.6) |
a Values are expressed as No. (%) or mean ± SD (min-max).
The patients were followed up at 1, 6, and 12 months and annually after the procedure. All data, including TTE reports, ECG, 24 hours Holter ECG, hemoglobin, and urinalysis tests, were recorded. Follow-up data from 380 (92%) patients were obtained regularly at a definite time, and 29 patients (8%) had some missing data.
3.1. Statistical Analysis
Statistical analysis was performed using Mann-Whitney U, Wilcoxon, Friedman, and Fisher’s exact tests whenever needed. SPSS 16 for windows (SPSS Inc., Chicago, Ill) was applied for statistical analysis, and a P-value < 0.05 was considered statistically significant.
4. Results
This study compared the results of percutaneous VSD closure between single-hole and multiple-hole perimembranous and muscular VSDs. It also evaluated the long-term follow-up (mean 48 months, range of 1 - 10 years) in 409 children (200 girls and 209 boys) who underwent transcatheter VSD closure.
The patients’ age was (2 - 15) 7 ± 1 years, and they weighed (10 - 75) 27.3 at the time of the procedure. The QP/QS rate was (1.4 - 4.5) 1.5 ± 0.5, the defect size measured by angiography was (4 - 15) 4.84 ± 2 mm, and the size of the implanted device was (4 - 14) 8 ± 2 mm.
Occlutech muscular VSD occluder (95 patients, 23.2%), muscular VSD of Lifetech (85 patients, 20.8%), and Asymmetric Membranous of Lifetech (60 patients, 14.7%) were used as the most frequent types of the device (Table 2).
| Device Type | No. (%) |
|---|---|
| Occlutech Muscular | 95 (23.2) |
| Muscular Lifetech | 85 (20.8) |
| Memb Lifetech Asymmetric | 60 (14.7) |
| Memb Lifetech Symmetric | 50 (12.2) |
| Coil pfm Le | 48 (11.7) |
| PDA Lifetech | 29 (5.9) |
| Occlutech memb | 18 (4.4) |
| PDA Occlutech | 10 (2.4) |
| Comed | 7 (1.7) |
| Cocoon | 3 (0.7) |
| Coil pfm PDA | 4 (1) |
| Total | 409 (100) |
4.1. Immediate Complications
Device embolization occurred in 3 patients. The embolized devices were recaptured in 2 patients, and VSD was percutaneous closed at the same course. The VSD of another patient was surgically closed. Arrhythmias were detected in 47 patients, hemolysis in 2 patients, mild tricuspid valve regurgitation in 45 patients, and mild aortic valve regurgitation in 1 patient after implantation or during the follow-up period. However, major complications, including procedure-related obstruction, cardiac tapenade, thrombosis, air embolism, or aortic rupture, were not observed. Moreover, no mortality was found in this study.
4.2. Residual Ventricular Septal Defect
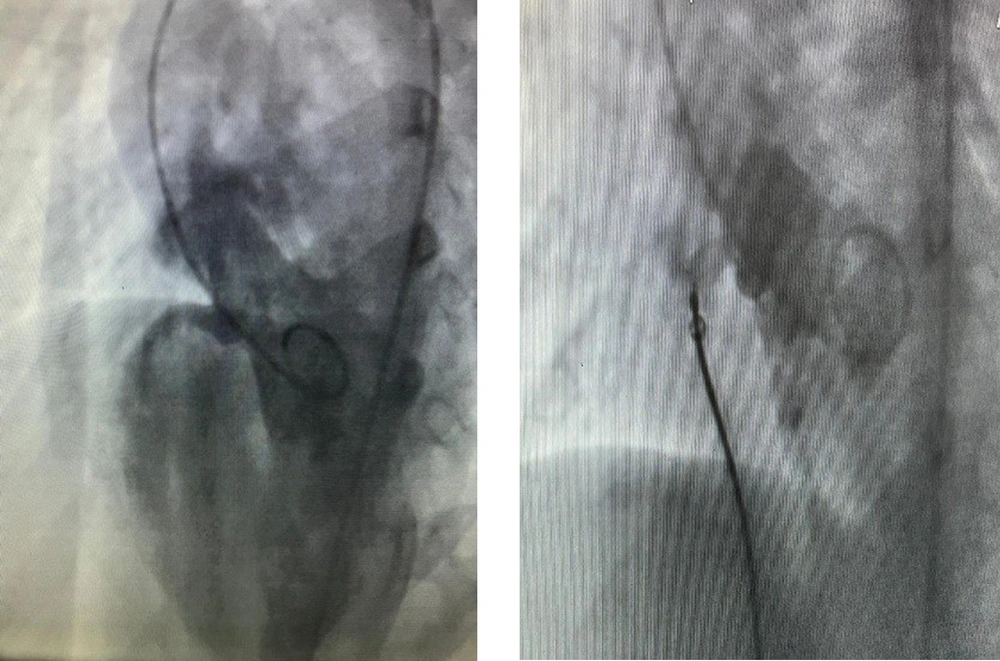
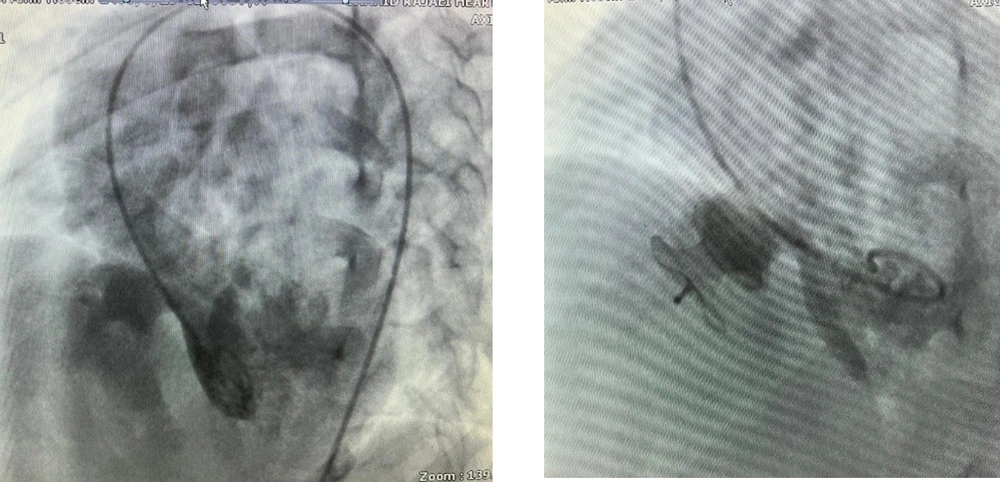
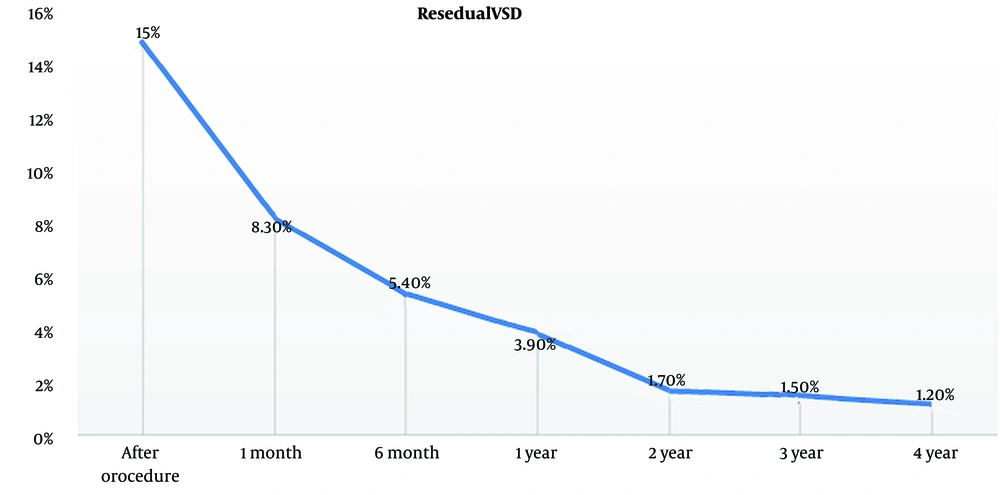
Residual VSD is defined as incomplete defect closure by the device, and it causes a minor blood shunt from the left to the right ventricle. In this study, we compared the rate of residual VSD in VSDs with a single hole and multiple holes (Figures 1 and 2).
During the procedure, the residual shunt in patients with a multiple-hole VSD was significantly higher than those with a single-hole one (P = 0.008). Patients with residual VSD were followed up using echocardiography, and the changes in the residual VSD shunt were also compared between patients with single-hole and multiple-hole VSDs. The residual shunt decreased over time in patients with a single-hole VSD (Figure 3).
4.3. Tricuspid and Aortic Valve Regurgitation
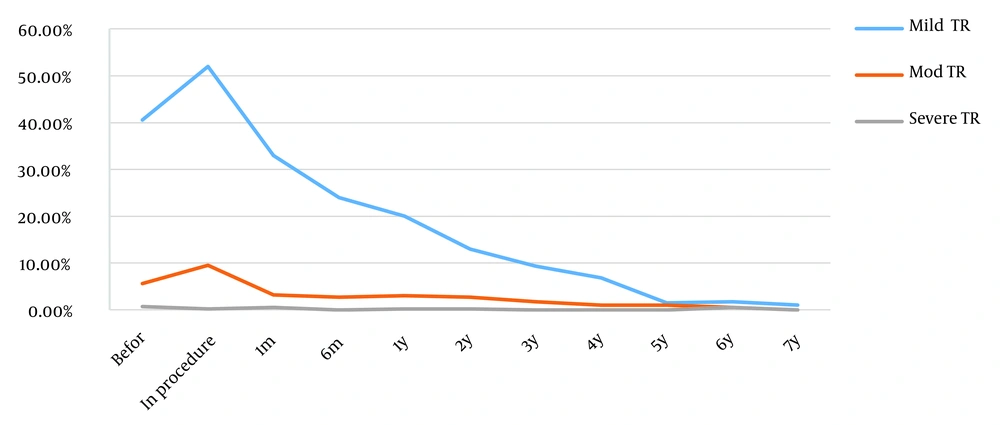
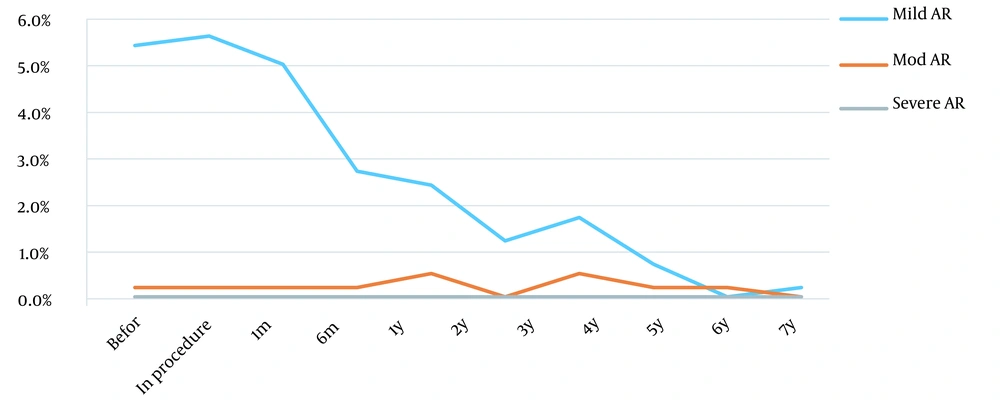
The incidence of new-onset tricuspid regurgitation (TR) and aortic valve regurgitation (AR) slightly increased during the procedure. However, the incidence of TR and AR significantly gradually decreased after the procedure (P < 0.001).
Mild TR was noted in 166 (40.6%) patients before the procedure and was observed in 211 (51.6%) patients during the procedure. It also persisted in 135 (33%), 99 (24%), and 81 (20%) patients 1, 6, and 12 months after the procedure, respectively. Forty-five patients (11%) had new onset of mild TR during the procedure, which was reduced dramatically over time (P < 0.001). Tricuspid regurgitation disappeared utterly in 5 years (Figure 4). Furthermore, 16 patients had new-onset moderate TR during the procedure that significantly disappeared after 1 year (P < 0.001). After the procedure, no patient was observed with a new onset of severe TR. Also, 22 (5.4%) patients had mild AR before the procedure, which was reduced to 10 (2.4%) patients after 1 year. Only 1 patient had new onset of mild AR during the procedure. No patient with new-onset moderate or severe AR was identified after the procedure (Figure 5).
Also, in this study, we compared different types of devices in creating new TR and aortic regurgitation.
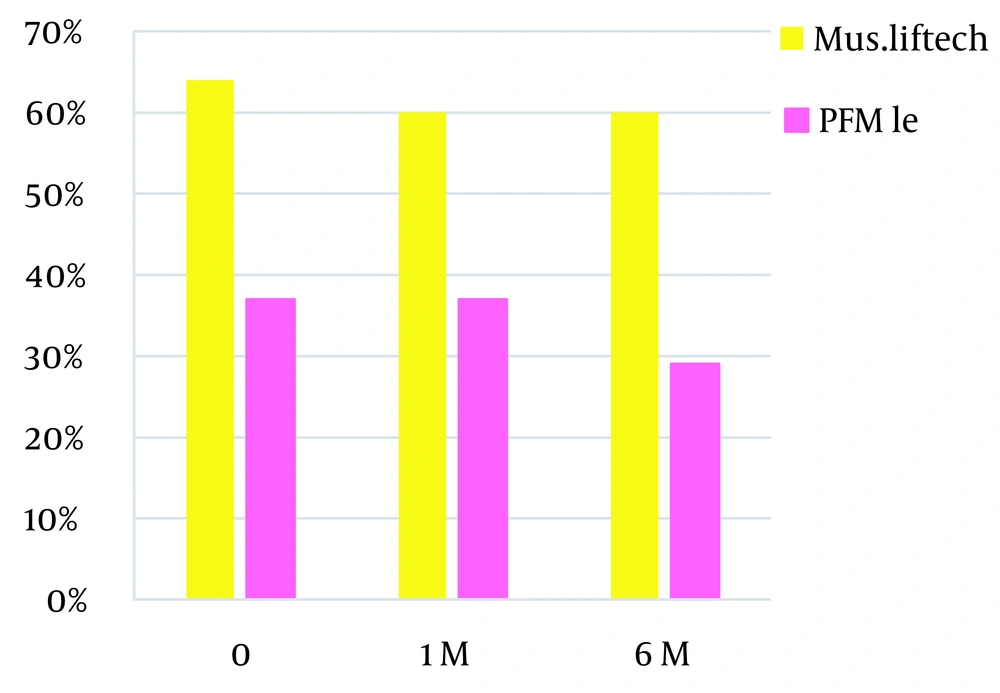
In comparing types of devices that cause new TR, it should be mentioned that muscular Lifetech has the most prevalence, while Pfm Lee has the least prevalence (Figure 6). This study showed no relationship between aortic regurgitation and device types (P = 0.675).
4.4. Arrhythmia
The most important complication was the complete atrioventricular block in 2 patients, which occurred 2 and 3 days after the procedure, and pacemaker implantation was performed for both of them after 10 days. A total of 45 patients (11%) had transient arrhythmia during the procedure that ultimately resolved during the first month. The most common arrhythmia was accelerated junctional rhythm in 34 patients (8.5%). It resolved with conservative therapy within a week to 1 month and returned to normal sinus rhythm. Five patients (1.2%) experienced atrial rhythm and atrial tachycardia (AT), and all returned to normal sinus rhythm within 2 weeks. Sinus node dysfunction (SND) occurred in 1 patient (0.2%), which resolved spontaneously before discharge. Also, bigeminal PVC occurred in 4 patients (1%) after the procedure, and the patient fully recovered after 3 days of steroid therapy. One patient had a complete right bundle branch block (RBBB) after the procedure, which reoccurred during the follow-up. The summary of arrhythmia after transcatheter VSD closure is shown in Table 3.
| Arrhythmia | n |
|---|---|
| Accelerated junctional rhythm | 34 |
| Atrial rhythm | 5 |
| Bigeminate PVC | 4 |
| Complete atrioventricular block | 2 |
| Complete right bundle branch block | 1 |
| Sinus node dysfunction | 1 |
| Total | 47 |
4.5. Hemolysis
Hemolysis developed in 2 patients (0.5%). A 4-year-old girl had the brown color of 24-hour urine after the procedure, and hemolysis occurred in a 5-year-old boy 1 month after the procedure, although it did not need a blood transfusion and was spontaneously resolved with conservative therapy. They did not have a residual shunt in the post-procedure echocardiography.
5. Discussion
Transcatheter VSD closure is a more complex and challenging procedure. It has potential complications such as device embolization, atrioventricular block (AVB), arrhythmia, new-onset tricuspid, aortic regurgitation, and hemolysis.
Cardiac arrhythmias have been identified as a joint event in transcatheter closure of Pm VSD due to the proximity of the conduction system to the perimembranous defect. An important point is observing the patient and immediately investigating any arrhythmias or hemodynamic change within 24 hours after the procedure (6). One potential and serious complication is a complete heart block, which is reported in 0.23 - 6.4% of the cases (7). It has also been reported that the squeezing effect of the device, an oversized device, direct compression, or late effects due to inflammation and fibrous tissue formation can result in a complete heart block (8). It took almost 1.5 years after ADO implantation in the selected cases to diagnose 1 complete atrioventricular block that required permanent pacing (9). For interventional treatment, complete AVB can be considered an early and late complication; however, in the surgical procedure, complete AVB usually is an immediate finding after the operation (10). According to this approach, with a high incidence of complete heart block, VSD device closure is not currently approved in the USA for perimembranous VSD closure. In many regions, including Europe and Asia, transcatheter VSD closure is the recommended method of choice in anatomically feasible and selected cases. In a study by Ghaderian et al., 28 patients underwent percutaneous Pm VSD closure using ADO with a mean follow-up period of 8.3 ± 3.6 months, and complete heart block, life-threatening arrhythmia, and death was not observed (9). In the present study, complete AVB occurred in only 2 patients. The first case was a 6-year-old girl with Pm VSD with complete AVB occurring 2 days after placing a 10 mm membranous Amplatzer device. The second one was a 6-year-old girl with high muscular VSD with complete AVB occurring 3 days after placing a 10 mm muscular Amplatzer device. Since the parents refused device removal, pacemaker implantation was performed for both of them within 10 days. Age below 6 years has been reported to be significantly associated with the risk of complete AVB (11), while others showed AVB mainly noted in less than 3 years of age group (12). In our cases, both patients were 6 years old.
Other transient arrhythmias were observed early and during the follow-up, which were treated with conservative treatment. Moreover, hemolysis is another serious complication that usually emerges immediately after the procedure. It may sometimes be transient, require medication, or require a blood transfusion (13). In the present study, hemolysis was observed in 2 patients 24 hours after VSD closure with coil Lee Pfm. In both of them, hemolysis resolved with conservative treatment. A blood transfusion was not performed in either case.
The new onset of valvular regurgitation, including aortic and tricuspid regurgitation, is well-known after the procedure. A study in China showed that new TR was seen in 5.4% of cases and did not require intervention (14). However, surgical intervention may require severe TR and progressive AR (15). Thus, transthoracic echo is crucial for pre-, intra-, and post-procedure monitoring. A new study by Li et al. among 253 patients showed new moderate tricuspid valve regurgitation in 4 patients (5). Valve regurgitation improved in all subjects without treatment at the 2-year follow-up (16). In the present study, 45 patients (11%) had new mild tricuspid valve regurgitation, and 16 patients (4%) showed new moderate tricuspid valve regurgitation after the procedure, which over time, was reduced dramatically (P < 0.001). Tricuspid valve regurgitation improved in all patients without treatment at 4-year follow-up. In our study, 22 patients (5.4%) had mild aortic regurgitation before the implantation of the device. New onset of mild aortic regurgitation was noted in 1 patient, which resolved during the next month. All cases of aortic regurgitation had resolved without treatment at the 2-year follow-up.
The mechanism of TR is not well known. It is probably a result of interference with chordae tendineae by the catheter, guide wire during the procedure, or the right disc of the device (17).
We compared different types of devices in creating new TR and aortic regurgitation. In comparing types of devices that cause new TR, it should be mentioned that muscular Lifetech has the most prevalence, while Pfm Lee has the least prevalence. Muscular Lifetech has a bigger right ventricle disc than pfm Lee. The greater prominence of the disc in the right ventricle can cause tricuspid regurgitation with a higher prevalence. This study showed no relationship between aortic regurgitation and device types (P = 0.675).
In a EUREVECO trial using the pfm-Lee-VSD coil, a trivial shunt was detected in 50% of patients immediately after the procedure (6).
In 2021, Bergmann et al. evaluated the long-term follow-up of 109 patients with percutaneous VSD closure (18). All residual shunts were small. They identified 2 risk factors for residual shunting: Ventricular septal defects after surgical closure and the use of the Nit-occlud devices. They did not compare residual shunting in mono-hole and multiple-hole VSDs, whereas we identified that multiple-hole VSDs were a risk factor for a residual shunt in the present study (19). Previous reports on the relevance between hole number and residual VSD were weak to our knowledge. We showed that the incidence of a residual shunt after the procedure in patients with a multiple-hole VSD was significantly higher than those with a single-hole VSD (P = 0.008). During the follow-up, the residual shunt decreased significantly within the first year of post-procedure, with great attention in the single-hole VSD group.
Overall, in our experience, no early and late significant complications such as mortality or endocarditis occurred. The most important complication was a complete heart block in 2 patients. Although TR and aortic regurgitation increased during the procedure, a significant decrease occurred during the follow-up. The transcatheter device closure of ventricular septal defects needs close follow-up by transthoracic echocardiography and electrocardiography.
5.1. Conclusions
We concluded that after transcatheter VSD closure, the residual shunt decreased dramatically after 1 year of follow-up, with a high rate in the group of patients with a single-hole VSD. Also, we showed that TR and AR dropped gradually.