1. Background
One of the most common causes of fever among children is urinary tract infection (UTI). Vesicoureteral reflux (VUR) is a situation in which urine flows reverse from the bladder to one or both ureters and, in some cases, to the kidneys and has been found in about 30 to 50% of children after the first episode of UTI (1). While most studies have reported the overall prevalence of VUR to be around 1% in the general population (2), 30 to 40% of siblings of affected children also suffer from the disease, indicating a familial involvement pattern (3). VUR is internationally classified into five grades: Grade I (reflux into the dilated ureter), grade II (reflux to the pelvis and without dilation), grade III (mild to moderate dilation of the ureter, kidney pelvis, and calyces with partial blunting of the fornix), grade IV (moderate ureteral tortuosity and dilation of the pelvis and calyces), and grade V (severe dilatation of the ureter, pelvis, and calyces with loss of papillary compressive effects and severe ureteral tortuosity) (4). Eventual renal scarring and renal failure resulting from recurrent UTIs are the most feared complications of prolonged and untreated VUR (5, 6). The incidence of renal scarring is low (about 15%) in patients with low-grade reflux. As the degree of reflux increases, the probability of renal scaring increases, so in grade IV or V of reflux, 65% of patients have renal parenchymal scars (7). Additionally, unilateral and bilateral VUR is associated with some congenital urinary tract developmental abnormalities, such as a posterior urethral valve (PUV) and ureterocele (8, 9). Voiding cystourethrogram (VCUG) and radionuclide cystography (RNC) are the primary diagnostic modalities to assess and grade VUR.
Few studies have shown a close association between VUR and gastroesophageal reflux disease (GERD) (10, 11). Although the occurrence of VUR and GERD is seemingly distinct in nature, the natural course and pathophysiology of these two entities (both VUR and GER are the results of sphincter dysfunction) resemble, as they both tend to improve simultaneously with age (12). GER, or gastroesophageal reflux, is a symptomatic digestive disorder. It happens when stomach contents and acid reflux into the esophagus. Physiological reflux is normal in infants under 8 to 12 months. GERD is treated after 18 months of age or if there are complications such as esophagitis, respiratory symptoms, or lack of appropriate weight gain in younger infants (13). The most common causes of GERD include transient relaxation of the lower esophageal sphincter (LES) or hiatal hernia (14). The most common complications of GERD are failure to thrive, recurrent bronchitis, and aspiration pneumonia.
The gold standard for diagnosing GERD is 24-hour manometry; however, this method is time-consuming and aggressive (13). Ultrasound is very sensitive in diagnosing GERD and assessing its severity and is readily available and non-invasive compared to 24-hour pH manometry (15). Ultrasound is also helpful in evaluating the effectiveness of GERD treatment approaches. Therefore, ultrasound is considered a practical and alternative method for the screening and follow-up of patients with GERD. In a study, after simultaneous examination of ultrasound and 24-hour manometry, the sensitivity and specificity of ultrasound for the diagnosis of GER were 100% and 87.5%, respectively (16).
2. Objectives
Considering the possibility of GERD in children with VUR and its significant consequences, it seems that the simultaneous study of these two common disorders in children might be clinically important. Furthermore, examining the relationship between the two phenomena can better understand the underlying pathophysiology of these common childhood disorders and find solutions for better therapeutic management. However, the limited number of studies in this field warns of the need for further research. Thus, the present study aimed to demonstrate a possible association between VUR and GERD among children.
3. Methods
This was a cross-sectional study on children aged between 1 and 14 years who were referred to a tertiary referral teaching hospital for VCUG between June 2019 and January 2020. One hundred thirteen children were referred for VCUG, but 31 were infants, 20 had exclusion criteria, and 62 children were assessed. The Ethics Committee at the TUMS approved this study. Written consent was obtained from the legal guardian of all patients regarding the VCUG and ultrasound procedures. All children had at least one clinical indication for VCUG assessment, including (1) a febrile urinary tract infection, (2) urinary tract malformations on ultrasonography, and (3) lower urinary tract dysfunction (17, 18). Those with active UTI or Posterior Urethral Valve (PUV) or the existence of any other anomalies of the urinary system, such as the ectopic urethra, ureterocele, separate urinary system, prune-belly syndrome, or neurogenic bladder, were excluded.
Each patient referred to the Radiology Department of our tertiary referral teaching hospital for VCUG was examined in terms of clinical indication for VCUG and then entered the study if relevant criteria existed. After insertion of a sterile catheter with a suitable size (Ch/Fr 8, 10 cm), the bladder was filled with the desired volume of water-soluble non-union contrast agent (visipaque). The expected volume of contrast material used to fill the bladder was calculated based on the following formula: Capacity (mL) = (2 + age [years]) × 30 (19). Scout bladder images were taken in anteroposterior/lateral oblique positions. Anteroposterior and oblique images were also captured during urination and after completely emptying the bladder to observe the evidence of VUR. If any exclusion criteria, including PUV or other urinary tract abnormalities, were detected during VCUG, the subject was excluded from the study. A radiologist with eight years of experience in pediatric imaging for the presence of VUR evaluated all VCUG results. Finally, patients with VUR were included as the cases and those without VUR as the controls. Afterwards, all subjects were assessed for GERD using a Philips AFFINITI 50 ultrasound device (Philips Deutschland, Hamburg, Germany) and by another pediatric radiologist who was blinded to the patients' VCUG results. The 3.5 MHZ curve probe was placed in the subxiphoid region at 5 and 11 o'clock, and then the gastroesophageal junction was examined for return of stomach contents to the esophagus for 15 minutes in B-mode. Significant return of gastric contents three times or more in 15 minutes was considered GERD.
For statistical analysis, the results are expressed as mean ± Standard Deviation (SD) for quantitative variables and summarized as frequencies (percentages) for categorical variables. Continuous variables were compared using either the t-test or the Mann–Whitney test when data did not follow a normal distribution or violated the assumption of equal variance between the study groups. Values of P ≤ 0.05 were considered statistically significant. The relative risk or risk ratio of GER in VUR was calculated. A risk ratio greater than 1.0 indicates an increased risk. SPSS 23.0 for Windows (IBM, Armonk, New York) was used for statistical analysis.
4. Results
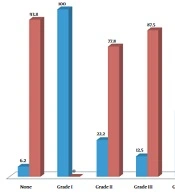
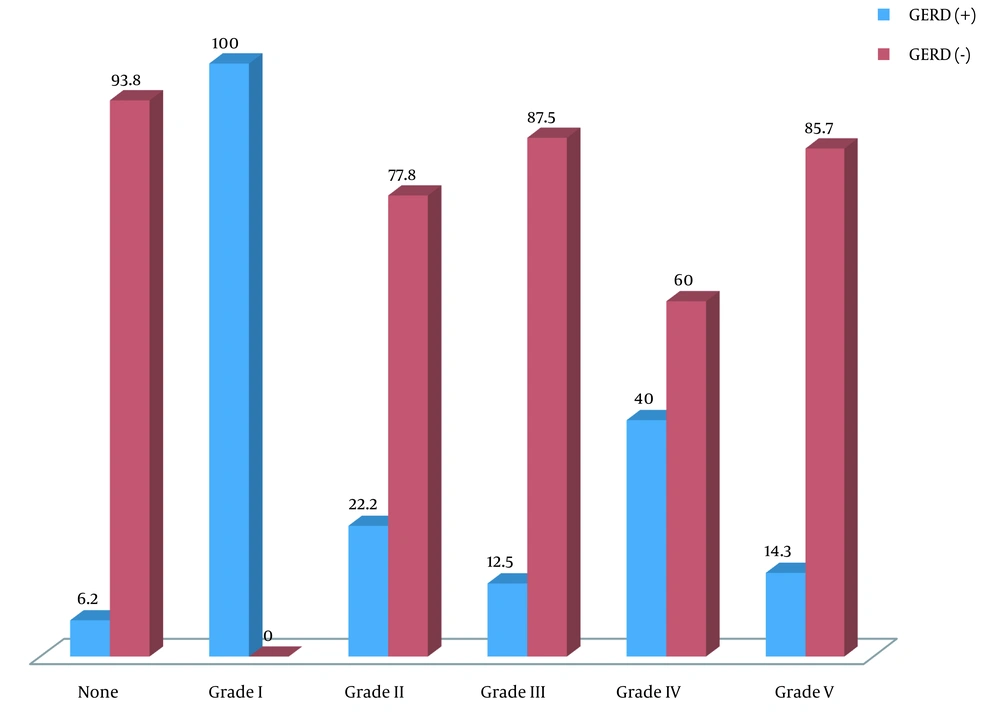
In total, 62 children candidates for VCUG were assessed. The mean age of the patients was 58.94 ± 38.83 months, ranging from 14 to 168 months, and 61.3% were female. Thirty-two patients were in the control group, consisting of 12 (37.5%) boys and 20 (62.5%) girls. The case group consisted of 30 children, 12 (40%) males and 18 (60%) females. Patients were divided into two subgroups: Age 48 months or younger and upper 48 months. According to the ultrasound assessment, 14.5% of the subjects suffered from GERD: 21.9% in the first and 6.6% in the second subgroups. The prevalence of GERD for different age subgroups among both genders in the case and control groups is depicted in Table 1. We found that the prevalence of GER reduces as the child grows. No relation was found between the first and second subgroups of age and the prevalence of GER (P = 0.059 and P = 0.133, respectively). Also, VUR was detected in 48.4% of children (40.0% in males and 60.0% in females) assessed by VCUG, of which 16.6% and 23.3% were graded as IV and V, respectively. Bilateral VUR was found in 46.6% of 62 children. Seven (23.3%) had concomitant VUR and GERD: 3.3% in boys and 20.0% in girls, indicating no difference between the two genders in the two subgroups (P = 0.41 and P = 0.37, respectively). There was no linear relationship between increasing age and the occurrence of VUR, GERD, or their concurrence. The prevalence of concurrent GERD and VUR was independent of gender or age. Also, 23 (76.7%) were diagnosed with VUR without any evidence of GERD. Only two (6.2%) patients had GERD without evidence of VUR. In the two groups with and without VUR, the prevalence of GERD was 23.3% and 6.2%, respectively, indicating a relative risk of 2 (95% confidence interval [CI]: 1.32 -3.02, P = 0.001). However, we found no association between the grade of VUR and the occurrence of GERD in the first and second subgroups (P = 0.194 and P = 0.839, respectively) (Table 2) and the total population (P = 0.137) (Figure 1). In the two groups with and without VUR, no difference was revealed in gender (60.0% versus 62.5%, P = 0.840), mean age (53.73 ± 38.80 months versus 63.81 ± 38.83 months, P = 0.311), or clinical findings (23.3% versus 25.0%, P = 0.878).
| Age | Sex | VUR | GER (+) | P-Value | |
|---|---|---|---|---|---|
| 0 - 48 mo | Female | Yes | 12 (37.5) | 5 (60) | 0.051 |
| No | 8 (25) | 0 | |||
| Male | Yes | 7 (22) | 1 (14.3) | 0.731 | |
| No | 5 (15.6) | 1 (20) | |||
| > 48 mo | Female | Yes | 6 (20) | 1 (16.6) | 0.56 |
| No | 12 (40) | 1 (8.3) | |||
| Male | Yes | 5 (16.6) | 0 | - | |
| No | 7 (23.3) | 0 | |||
a Values are expressed as No. (%).
| VUR Grade | GER, No. (%) | |||
|---|---|---|---|---|
| 0 - 48 Months | > 48 Months | |||
| No | Yes | No | Yes | |
| Negative | 13 (92.9) | 1 (7.1) | 17 (94.4) | 1 (5.6) |
| Grade 1 | 0 (0.0) | 1 (100.0) | 0 (0) | 0 (0) |
| Grade 2 | 5 (83.3) | 1 (16.7) | 2 (66.7) | 1 (33.3) |
| Grade 3 | 3 (75.0) | 1 (25.0) | 4 (100.0) | 0 (0) |
| Grade 4 | 1 (33.3) | 2 (66.7) | 2 (100.0) | 0 (0) |
| Grade 5 | 4 (80.0) | 1 (20.0) | 2 (100.0) | 0 (0) |
5. Discussion
The pathophysiological changes that prevent Ureterovesical Junction (UVJ) from adequate closure and make it susceptible to VUR include the loss of smooth muscle neural and vascular cells, collagen deposition, and eventual extensive fibrosis in the sub-mucosal layer of the valve. Schwentner et al. suggested that the basis for these developmental pathohistological abnormalities may lie in the gene expression of smooth muscle cells or proteomes (17).
Similarly, Meneghetti et al. have proposed that underlying pathological mechanisms for GERD are muscle damage in the lower esophageal sphincter or abnormal neural control of this sphincter that leads to its incompetence (19). Due to the concurrent occurrence of VUR and GERD in a significant number of children and the fact that improving one of the diseases can improve another and vice versa, the pathophysiological basis of the two diseases may likely be linked together, and genetics of smooth muscle cells are probably involved.
In the present study, among all children who were candidates for VCUG due to clinical indication, about one-ninth concurrently suffered from VUR and GERD, indicating a significant association between the two entities. Presumably, by employing larger sample sizes, this association could be demonstrated with more statistical power.
A few studies have assessed the concurrence of VUR and GERD and their underlying pathophysiological and developmental mechanisms. As recently shown by Hosier et al. (10), of the 404,300 confirmed patients, 6.6% were diagnosed with GERD, 0.33% with VUR, and 0.08% with both, indicating lower rates of such phenomena compared to our study. This study was a retrospective study of patient records, while our study was prospectively designed. However, similar to our study, the prevalence of GERD in patients with VUR was 24.3% compared to 6.6% in patients without VUR. Therefore, those with GERD had a higher risk of being diagnosed with VUR. In another study, Pooli et al. (11) demonstrated that GERD was more common in patients with primary VUR and those with advanced VUR. They also showed that the co-occurrence of GERD and VUR was more likely in younger children, and thus it seems that the search for underlying pathophysiological etiology of this phenomena in younger children might be more reasonable. In other words, such an association can be better sought in the physical and clinical changes of children in the stages of growth and development, as well as metabolic changes with age, because such a relationship might be weakened with the child's growth. Although our study was performed in older patients, VUR could be a risk factor for GER, similar to this study. As we had limitations for VCUG, the small sample of children and wide age range caused the heterogeneity of the variables. We suggest a new study with a larger sample size in two subgroups by age younger than 12 months with physiological GER and older children and evaluate the concordance of GER and VUR.
In conclusion, if we exclude children with physiological GER from the study, the children with VUR are three times more likely to have GERD than children without VUR. This possible association implies a likely intertwined underlying pathophysiological or embryological etiology, which still needs to be fully explored.