1. Background
Nocturnal enuresis refers to involuntary urination during nighttime sleep in children after age 5 (1-3). These problems can cause social and psychological issues for children and their parents if left untreated (4, 5). Monosymptomatic and nonmonosymptomatic enuresis are two types of enuresis. About 80% of enuresis is monosymptomatic, with no other lower urinary tract symptoms and bladder dysfunction symptoms. If the child shows signs and symptoms of lower urinary tract dysfunction, such as urgency, frequency, and so on, it is known as nonmonosymptomatic enuresis (2, 3). Enuresis is defined as primary when there has been no achievement in nighttime dryness since the beginning and secondary when it has occurred after at least six months of dryness. Inadequate antidiuretic hormones, sleep problems, and detrusor overactivity at night are some pathogenesis factors for primary monosymptomatic nocturnal enuresis (PMNE). Bedwetting alarms, tricyclic antidepressants, and desmopressin are treatment modalities for this condition (2, 3, 5-7).
With regard to the numerous etiologies of enuresis, researchers are trying to find new biomarkers to better evaluate the pathogenic factors, the prognosis for recovery, and the best treatment decisions in primary monosymptomatic enuresis. Desmopressin is one of the common treatments for nocturnal enuresis, but 20 - 60% of monosymptomatic enuresis have no response to this drug. Few studies suggest that a night release of antidiuretic hormone (ADH) might be determined the responsiveness to desmopressin (8, 9). Antidiuretic hormone or arginine vasopressin (AVP) is a neurohypophyseal hormone that controls osmotic homeostasis; it conserves water and increases urine concentration, which reduces urine volume (10). In children, AVP measurement is rarely used clinically. In addition, plasma AVP concentrations are unreliable by many laboratory assays because more than 90% of circulating AVP binds to platelets and clears rapidly (10, 11).
Copeptin is the C-terminus of pre-vasopressin (pre-pro AVP) and is a new neurohormone from the AVP10 system. Copeptin is a glycosylated peptide containing 39 amino acids with a leucine-rich core fragment having a molecular mass of 5 kDa (10, 11). Copeptin is stored in pituitary nerve vesicles along with AVP and neurophysin II until it is secreted (12). Copeptin is synthesized with AVP and is a stable fragment of AVP precursor, and its amount is equivalent to AVP in the bloodstream of healthy and diseased people, and there is a high correlation between them. This biomarker could be applied to body fluid homeostasis disorders that depend on vasopressin (13-18). In contrast to AVP, copeptin measurement needs a small sample, and the technical procedure is easier; it is very stable with less than 20% loss of recovery at room temperature for 7 days and at 4 degrees centigrade for 14 days (15).
Due to the favorable structural properties of copeptin and the reflection of AVP concentration, it can be used as an alternative marker for AVP secretion in clinical conditions (19). There is some research about the role of copeptin in various disorders like myocardial infarction, pulmonary embolism, stress, and metabolic and neurologic diseases (20-22), but the study of the relation between copeptin and enuresis is limited. Only a few surveys studied the role of copeptin in enuresis, and there was controversy about the results of these researches (9). Nalbantoglu et al. found that enuresis children have a lower level of copeptin than healthy ones (4). Hara et al. demonstrated that the copeptin day/night ratio could predict desmopressin response (10). On the other hand, Szymanik-Grzelak et al. reported that there wasn’t any relation between copeptin and enuresis (9).
2. Objectives
Considering the role of changes in ADH secretion in children with enuresis and also the desirable characteristics of copeptin as a biomarker for diagnosis and treatment of enuresis; on the other hand, controversy about the results of the few studies conducted about copeptin and enuresis; the researchers decided to conduct a study to investigate the relationship between copeptin level and enuresis in children aged 5 - 15 years in Taleghani hospital in Gorgan.
3. Methods
This study was a case-control study. The population included children with confirmed monosymptomatic enuresis and children without enuresis referred to the pediatric clinic of Taleghani hospital in Gorgan in 2020.
Inclusion criteria included children aged 5 - 15 years with primary mono-symptomatic enuresis and healthy children aged 5 - 15 years who had been referred to the clinic of Taleghani hospital with a normal ultrasonography and creatinine level. Exclusion criteria included children with diurnal urinary symptoms, bladder dysfunction, secondary enuresis, cirrhosis, diabetes mellitus, obesity, metabolic syndrome, congenital heart or hematological diseases, and infection. None of the participants have taken medical drugs and vitamins in the last three months.
All children with the diagnosis of monosymptomatic enuresis, which was confirmed by the pediatric nephrologist, entered the study. Written consent was obtained from all the parents.
Blood samples were taken from all the participants to evaluate the level of copeptin. Blood samples were collected in pre-cooled tubes containing ethylenediaminetetraacetic acid (EDTA) and then plasma was removed and immediately frozen at -20°C until the measurement. The copeptin measurement was done by the kit made in Australia. We used the US-made enzyme-linked immunosorbent assay (ELISA) stat fax reader for the ELISA method of measurement.
3.1. Statistical Analysis
Continuous variables were summarized as mean ± standard deviation, and categorical variables were presented as numbers and percentages. Age was considered continuous and categorized into two groups (5 - 9 years and 10 - 15 years). Shapiro-Wilk test was used to test the normality of data distribution. The non-parametric Mann-Whitney test was used to compare the level of copeptin in the two groups and subgroups by sex and age categories. The statistical analysis was performed using SPSS software (version 21.0). A P-value < 0.05 was considered statistically significant.
The sample size was calculated for the equality test of the mean of Copeptin in two independent groups and based on the study of Nalbantoglu et al. (4), according to the estimate of the mean and standard deviation of copeptin in the group of children with and without enuresis, 3.74 ± 1.44, 16.57 ± 3.91 pg/mL, respectively and assuming a mean difference (precision) equal to 3 units instead of 12.83 and at the level of significance (α = 0.05) and a power of the test (1-β = 0.9), respecting the effect of sex, 42 people from each group and a total of 84 people were selected.
4. Results
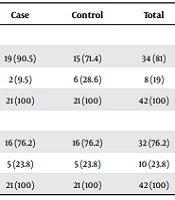
From 84 patients in equal proportions of 42 enuresis and healthy children, girls (n = 21) and boys (n = 21) participated in both enuresis and control groups. The mean and standard deviation of the total age of the children participating in this study was 8.05 ± 2.46 years, and the median was 7 years. The mean age in the enuresis group was 7.67 ± 2.26 years, and in the control group was 8.43 ± 2.62 years. The two groups had no statistically significant difference (P = 0.16). In this study, 34 girls (81%) and 32 boys (76.2%) were 5 - 8 years old (Table 1).
| Sex and Age (y) | Case | Control | Total |
|---|---|---|---|
| Girl | |||
| 5 - 8 | 19 (90.5) | 15 (71.4) | 34 (81) |
| 9 - 15 | 2 (9.5) | 6 (28.6) | 8 (19) |
| Total | 21 (100) | 21 (100) | 42 (100) |
| Boy | |||
| 5 - 8 | 16 (76.2) | 16 (76.2) | 32 (76.2) |
| 9 - 15 | 5 (23.8) | 5 (23.8) | 10 (23.8) |
| Total | 21 (100) | 21 (100) | 42 (100) |
a Values are expressed as No. (%).
The mean copeptin level in the case and control groups was 6.7 ± 4.27 and 6.87 ± 8.52 pg/mL, respectively. The median and interquartile range (IQR) in the enuresis and control groups were 5.35 (4.10) and 4.25 (1.67), respectively (Table 2). As shown in Table 2, the mean level of copeptin in the enuresis group was significantly lower than in the control group (P = 0.03).
| Groups | Number | Mean ± SD | Median (IQR) | Minimum | Maximum | P-Value |
|---|---|---|---|---|---|---|
| Enuresis | 42 | 6.7 ± 4.27 | 5.35 (4.10) | 2.9 | 23.1 | 0.03 a |
| Control | 42 | 6.87 ± 8.52 | 4.25 (1.67) | 2.9 | 43.7 |
Abbreviations: SD, standard deviation; IQR, interquartile range.
a Mann-Whitney test.
The data were classified into 2 age groups (5 - 9 years and 10 - 15 years) to compare the mean copeptin level by age. In both age groups, there was a statistically significant difference between the level of copeptin in the enuresis and the control groups (Table 3).
| Groups | Number | Mean ± SD (pg/mL) | Median (IQR) | Minimum | Maximum | P-Value |
|---|---|---|---|---|---|---|
| Age (y) | ||||||
| 5 - 9 | 0.047 a | |||||
| Case | 35 | 6.38 ± 4.10 | 4.60 (4.00) | 2.9 | 23.1 | |
| Control | 31 | 7.19 ± 6.24 | 3.80 (1.40) | 2.9 | 38.7 | |
| 10 - 15 | 0.059 a | |||||
| Case | 7 | 8.31 ± 5.05 | 6.90 (3.20) | 4.3 | 19.30 | |
| Control | 11 | 8.66 ± 11.72 | 4.90 (3.20) | 3.10 | 43.7 | |
| Sex | ||||||
| Female | 0.35 a | |||||
| Case | 21 | 6.97 ± 4.88 | 5.20 (4.75) | 2.9 | 23.10 | |
| Control | 21 | 7.47 ± 8.54 | 4.30 (2.55) | 2.9 | 38.7 | |
| Male | 0.03 a | |||||
| Case | 21 | 6.43 ± 3.65 | 5.40 (3.70) | 3.10 | 19.30 | |
| Control | 21 | 6.28 ± 8.66 | 4.20 (1.35) | 2.9 | 43.7 |
Abbreviations: SD, standard deviation; IQR, interquartile range.
a Mann-Whitney test.
In both the case and control groups, the mean level of copeptin is lower in children younger than 10 years than in those older (Table 3).
Table 3 shows the mean level of copeptin in each group of males and females. As is shown in the female group, the mean level of copeptin in the enuresis group was less than that in the control group, but it was not statistically significant (P = 0.35).
On the other hand, in the male group, the mean level of copeptin in the enuresis group was significantly lower than that in the control group (P = 0.03).
5. Discussion
Nocturnal enuresis in children is a common concern for both pediatricians and parents. Some treatments have been introduced to solve these issues, but their efficacy is debated. One of the common treatments for nocturnal enuresis is desmopressin, which is used because of the theory of an insufficient increase in ADH at nighttime. A vasopressin analog like desmopressin is prescribed to treat enuresis, but the responsiveness to this drug is insufficient (8, 9).
Arginine vasopressin, or ADH, regulates the homeostasis of fluids released from the pituitary into circulation. Some studies worked on the AVP in plasma as a biomarker for diagnosing and treating diseases associated with stress and fluid disorders (18). On the other hand, clinical assessment of AVP for a short half-time, instability, and technical reasons are so difficult. Copeptin is as equimolar as AVP and a reliable surrogate of AVP; the most advantages of this biomarker are the small amount of serum or plasma required and its very stable in plasma (15-17). The role of this new biomarker is well known in some diseases like cardiovascular, chronic obstructive pulmonary disease (COPD), and neurological and metabolic disorders (18, 22), but the relationship between this new marker and enuresis is under debate. Only a few studies assessed the role of copeptin in enuresis, and the results showed controversy. Hara et al. reported that copeptin has predictive value for desmopressin responsiveness, although studies are quite limited in this area to be compared with this research (10).
In this study, the mean level of copeptin in the case and control groups were 6.7 ± 4.27 and 6.87 ± 8.52 pg/mL, respectively, which was significantly lower in the case group (P = 0.03), which is in agreement with the study by Nalbantoglu et al. (4) and Girisgen et al. (1). Nalbantoglu et al. studied 88 enuresis children and healthy ones between 6 - 14 years old and showed that the level of copeptin was significantly lower in the enuresis group; that was the first report about this assessment (4).
Girisgen et al. studied 119 patients with enuresis that was 49 patients with monosymptomatic enuresis, and 40 healthy children 5 - 16 years that showed serum copeptin levels were significantly lower in comparison with healthy children and introduced that copeptin could be a surrogate biomarker of AVP. The authors stated that their study was the third article on this topic (1).
Hara et al. demonstrated that plasma copeptin level could be a predictive biomarker for evaluating desmopressin response. This study was conducted on 32 children with PMNE between 6 and 11 years old, the first study on this topic (10).
On the other hand, Szymanik-Grzelak et al. explained that there is no relationship between PMNE and copeptin level and showed that the role of copeptin as a reliable biomarker is under debate. It was a study of 25 children with the clinical diagnosis of PMNE between 5 - 15 years old, and the authors stated that assessment of the relationship between copeptin and enuresis required more studies (9).
In this study, there was a significant relationship between age and copeptin level, but Nalbantoglu et al. demonstrated that there was no relationship between this parameter, as is seen in Szymanik-Grzelak et al. article (4, 9). The mean age of the Nalbantoglu et al. study was 9.70 ± 2.04 compared with our study, which was 8.05 ± 2.46 years (4). In this study, in both age groups, there was a statistically significant difference between the level of copeptin in the enuresis and the control groups; however, the mean level of copeptin in children younger than 10 years is less than that in older children in both the case and the control group. It is recommended that further studies be designed to focus on the level of copeptin in different age groups.
According to our study in the female group, the mean level of copeptin in the enuresis group was less than that in the control group; however, it was not statistically significant (P = 0.35). Although in the male group, it was statistically significant (P = 0.035). In the study of Nalbantoglu et al. and Szymanik-Grzelak et al., there was no significant difference between the sex and the copeptin level (4, 9). In this study, the mean of copeptin level is not different between males and females as shown in Szymanik-Grzelak et al. and Skrzypczyk et al. and Przybylowski et al. study, but according to some studies, copeptin level in males is higher than in females (9, 23-26).
This study has some limitations; some confounders exist in enuresis, such as family history, sleep status, constipation, caffeine intake, sodium, and potassium, which were not assessed. Moreover, the sample size is small. It is recommended that a prospective study with a larger number of cases and control with proper sample size in sex and age subgroups be designed. The confounder item should be mentioned. Patients with a low level of copeptin should be treated with desmopressin, and the effectiveness of the treatment should be evaluated.
5.1. Conclusions
In this study, the mean level of copeptin in PMNE was significantly lower than that in the control group, which suggests it may be considered as a probable biomarker for the prediction of response to treatment with desmopressin, but further study, especially before and after desmopressin therapy in patients with a low level of copeptin is required to confirm this hypothesis.