1. Background
Constipation is a major problem in children, generally accompanied by decreased frequency of bowel movements, hard and large diameter stools, fecal incontinence, painful excretion of feces, and abdominal pain (1). In about 95% of pediatric patients with constipation, no intrinsic causation can be recognized; these are referred to as affected with functional constipation (FC) (2). The true prevalence of this problem is unknown in children, but various studies show that it varies from 0.7 to 29.6% (3-5). Children with FC make up 5% of all referrals to pediatricians and about 10 to 25% to pediatric gastroenterologists (6). It is more common in girls than boys (ratio 2.1: 1) (7). This disorder may lead to patient and family discomfort and impact their quality of life (8). Various factors can be involved in this problem: Socioeconomic status, consumption of low-fiber foods, inactivity, inadequate fluid intake, and genetic predisposition (9). Although several etiologies are blamed for constipation in the pediatric group, FC is diagnosed just as no organic pathology or red flag is predicated (10-12).
Treatment of FC begins with non-pharmacological interventions (toilet training, increasing fluid, and dietary fiber, and increasing physical activity and biofeedback therapy) and pharmacological interventions, including osmotic laxatives such as liquid paraffin and polyethylene glycol (PEG).
We designed this study to compare Jojoba paste with PEG as laxative drugs in children affected with FC. Jujube was selected by studying the texts of traditional Iranian medicine because it is native to our country, it is easy to access, its taste is acceptable to children, it is safe even in infants, and its extract has been used safely in the treatment of neonatal hyperbilirubinemia (13).
Jujube (Ziziphus jujuba Mill), also named Annab in Persian and Arabic language, is a medicinal tree belonging to the jujube genus (Rhamnaceae) that is distributed in different regions of Iran (14). It has been cultivated in China since 4000 years ago and is also known as Chinese date. In Oriental medicine texts, especially in China and Korea from 2500 years ago, its fruit, seeds, and peel have been used to treat insomnia, loss of appetite, indigestion, and arthritis and as a contraceptive. The jujube fruit is considered a healthy food, and its active ingredients are vitamin C, phenolics, flavonoids, triterpenic acids, and polysaccharides. Proteins, fibers, and minerals, namely phosphorus, potassium, calcium, magnesium, and iron, also comprise its pulp. Jujube fruit is a source of vitamins other than vitamin C, such as thiamin, riboflavin, niacin, vitamin B6, and vitamin A. Its fruits have anti-inflammatory effects, sedative activity, and gastrointestinal protective activity; it is an antioxidant, antifungal, and anti-cancer compound that enhances the body's immunity (15-17).
2. Objectives
As far as the authors acquaint, no study has been performed to assess the therapeutic effect of Jujube extract or paste in the treatment of FC in children, and this study is the foremost clinical trial to evaluate the effectiveness of this drug compared to routine drugs for treating FC in children. Therefore, we decided to conduct the present study to determine the effect of the Jujube oral product compared with the standard product for treating FC in children and to introduce a product with fewer side effects and significant nutritional value for children whose raw materials are available naturally in Iran.
3. Methods
3.1. Study Design
In this randomized controlled clinical trial, the target population included children with FC aged 2 to 12 years referred to the pediatric gastroenterology clinic of Rasoul-e-Akram Complex Hospital from May to November 2018.
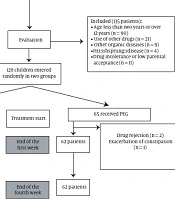
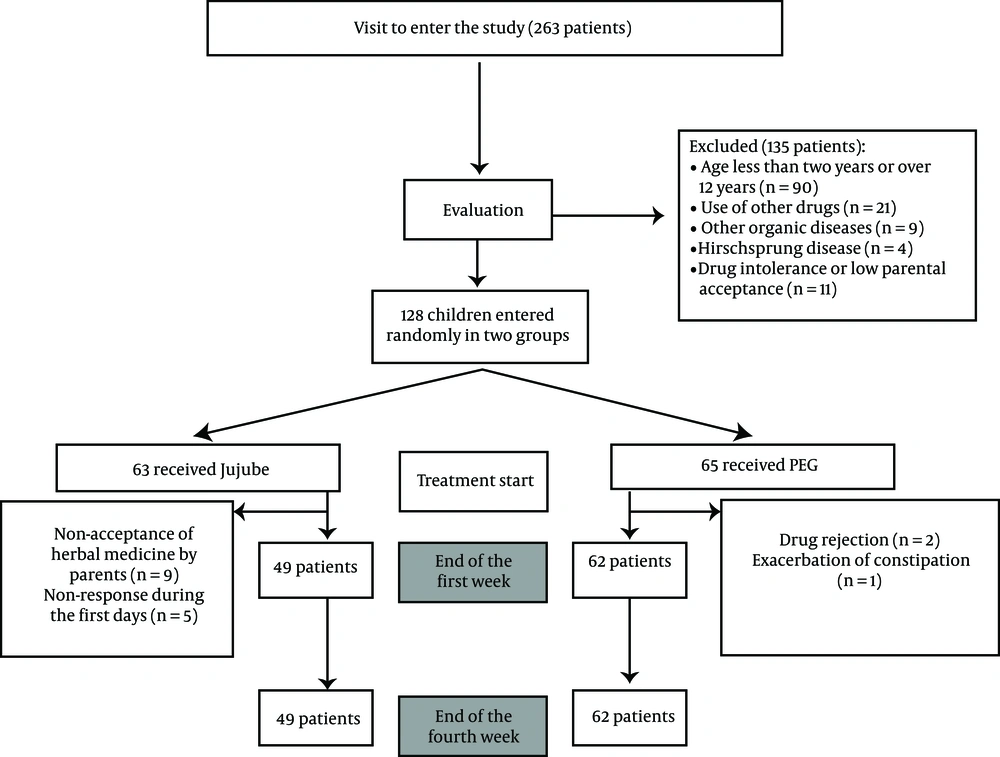
Based on a power of 84% and a critical significance level of 0.05, the sample size was calculated as 55 in each group and 110 in total. Considering a dropout rate of 15%, the authors recruited 128 children with FC based on Rome IV criteria which met the inclusion criteria.
The authors used Rome IV criteria for the definition of FC: Two or few bowel movements per week, at least one course of fecal incontinence or soiling per week, history of withholding or retentive posturing, history of hard or painful stool, presence of a fecal mass in the rectum, and history of large-diameter stool blocking the toilet. The presence of at least two criteria for at least one month, without sufficient criteria for diagnosing irritable bowel syndrome, is diagnostic for FC in children. It should be remarked that a few cases have all the Rome IV criteria for FC; accordingly, having defecation problems for two weeks or more, which causes stress for patients and their parents, is diagnostic for pediatric FC (18).
Among 128 children in the study, 62 patients from the PEG group and 49 patients from the Jujube group remained until the end of the study and were treated for four weeks. The study evaluated the jujube oral product composition's efficacy, safety, and tolerability. Patients entered the study after receiving oral explanations and obtaining written consent (from parents).
Patients were systematically divided into two groups randomly. Jujube paste was given to one group and PEG to the other group. The groups were explained how to use them. In both groups, demographic characteristics, including age and sex, as well as dependent variables, including patients' symptoms according to Rome IV criteria, patient acceptance rate, and intervening variables, including duration of disease, the onset of symptoms, fiber intake, and the presence or absence of fecal impaction was assessed. The painful and hard stool was measured according to the Visual Analogue Scale (VAS) as a measurement instrument in which the most comfortable state of defecation with no pain in the child was scored 0, and the most painful and the hardest state of defecation was scored 100.
To prepare jujube paste, dried jujube fruits were halved, crushed, and soaked in water for 10 hours. After that, the resulting extract was heated and strained. The resulting extract was exposed to gentle heat in the next step until its volume reached half and became firm (jujube paste). Jujube product at 0.5 cc/kg was administered twice daily to children in the patient group according to traditional medicine texts, particularly the Gharabadin book. The laxative used in the control group was PEG (Pidrolax; polyethylene glycol (PEG3350/4000) powder manufactured by Sepidaj company) at a dose of 0.4 g/kg/day.
Inclusion criteria for children were 2 to 12 years of age and having FC based on Rome IV criteria approved by a pediatric gastroenterologist. Exclusion criteria in this study were diseases such as significant psychiatric disorders, mental retardation, chronic medical diseases, developmental disorders, chronic gastrointestinal disorders or congenital anomalies, history of bowel and stomach surgery, use of medical treatment affecting GI movement, presence of organic constipation, intolerance to medication and possible side effects, incorrect use of medication, and discontinuing of treatment by the patient.
3.2. Statistical Analysis
Descriptive statistics are used for qualitative variables in frequency tables and bar graphs. We used mean and standard deviation (mean ± SD) for quantitative variables. Chi-square or Fisher test was used to examine the relationship between two qualitative variables. Information on weight before and after consumption, clinical symptoms, and the complication caused by the drug was obtained from the forms completed by the parents. Data obtained from the two groups of patients were compared before enrollment, in the second week, and at the end of the fourth week of the study.
First, normality was checked using the Kolmogorov-Smirnov test (P-value < 0.05). Non-parametric tests such as the Human-Whitney test were utilized to compare the means of the two groups. The Friedman test was used to compare the means over time. Data analysis was conducted using SPSS 24 at a significance level of 0.05.
The Ethics Committee of the Iran University of Medical Sciences approved the study with the code of IR.IUMS.REC 1396.9421309003. The registration code of the study as a clinical trial in IRCT is IRCT20171213037866N1 (https://en.irct.ir/trial/30614).
4. Results
Between May and November 2018, 128 patients with FC aged 2 to 12 years (mean of 4.2 ± 2.7 years) were included in the study. The number of people included in the study and the different stages of this work are summarized in Figure 1. Demographic data and clinical characteristics of children in the case and control groups are listed in Table 1.
| Variables | PEG Group | Jujube Extract Group | P-Value |
|---|---|---|---|
| Age, y | 4.7 ± 2.5 | 5.3 ± 2.8 | 0.29 |
| Gender | 0.71 | ||
| Male | 28 (45.2) | 24 (49) | |
| Female | 24 (54.8) | 25 (51) | |
| Weight before consumption, kg | 19.2 ± 10.08 | 20.07 ± 9.07 | 0.06 |
| Defecation frequency | 2.64 ± 2.12 | 3.02 ± 2.38 | 0.86 |
| Stool stiffness | 82.58 ± 13.3 | 87.14 ± 10.8 | 0.06 |
| Painful defecation | 71.29 ± 33.44 | 67.14 ± 37.53 | 0.91 |
| Stool withholding | 8.67 ± 5.3 | 8.16 ± 6.14 | 0.78 |
| Large diameter stool | 2.83 ± 3.04 | 2.69 ± 2.19 | 0.75 |
| Soiling | 1.68 ± 2.47 | 2.8 ± 5.39 | 0.97 |
Demographic Data and Clinical Characteristics of Children with Functional Constipation in Two Groups of Polyethylene Glycol and Jujube
The weight of people in the Jujube extract group increased slightly after the drug use (21.07 ± 9.07 to 21.72 ± 8.75 kg). Also, the weight of people in the PEG group increased after drug consumption (17.18 ± 9.55 to 18.64 ± 9.54 kg). However, patients' weight gain was not statistically significant after treatment in both groups of Jujube and PEG.
All criteria of FC in the Jujube and PEG groups before, two, and four weeks after treatment are compared in Table 2. These findings indicate that in all cases studied, improvement was achieved in both groups.
| Clinical Characteristics and Group | Before Treatment, Mean ± SD | Two Weeks, Mean ± SD | Two Weeks, % | Two Weeks P-Value | Four Weeks, Mean ± SD | Four Weeks, % | Four Weeks P-Value |
|---|---|---|---|---|---|---|---|
| Defecation frequency | 0.887 | 0.927 | |||||
| Jujube | 3.02 ± 0.34 | 6.88 ± 0.41 | 128 | 7.14 ± 0.36 | 136 | ||
| PEG | 2.64 ± 0.26 | 6.76 ± 0.39 | 155 | 6.74 ± 0.28 | 155 | ||
| Stool stiffness | 0.019 | < 0.0001 | |||||
| Jujube | 87.14 ± 1.54 | 41.02 ± 3.47 | 55 | 27.35 ± 2.71 | 69 | ||
| PEG | 82.58 ± 1.68 | 46.61 ± 2.82 | 42 | 38.38 ± 1.89 | 53 | ||
| Painful defecation | 0.798 | 0.920 | |||||
| Jujube | 67.14 ± 5.36 | 17.35 ± 3.87 | 76 | 12.85 ± 2.91 | 81 | ||
| PEG | 71.29 ± 4.24 | 24.19 ± 4.03 | 66 | 17.26 ± 3.05 | 76 | ||
| Stool withholding | 0.944 | 0.944 | |||||
| Jujube | 8.16 ± 0.87 | 1.86 ± 0.50 | 77 | 1.08 ± 0.34 | 87 | ||
| PEG | 8.67 ± 0.67 | 2.39 ± 0.55 | 72 | 1.56 ± 0.42 | 82 | ||
| Large diameter stool | 0.087 | 0.076 | |||||
| Jujube | 2.69 ± 0.31 | 0.75 ± 0.22 | 72 | 0.24 ± 0.11 | 91 | ||
| PEG | 2.84 ± 0.38 | 0.9 ± 0.19 | 68 | 0.66 ± 0.11 | 77 | ||
| Soiling | 0.940 | 0.915 | |||||
| Jujube | 2.86 ± 0.77 | 1.53 ± 0.48 | 46 | 1.18 ± 0.36 | 59 | ||
| PEG | 1.68 ± 0.31 | 0.74 ± 0.33 | 56 | 0.64 ± 0.27 | 61 |
Comparison of Clinical Characteristics of Children with Functional Constipation Before, Two, and Four Weeks After Treatment by Jujube and Polyethylene Glycol
The defecation frequency in the Jujube extract group changed from 3.02 ± 2.38 per week before inclusion to 6.88 ± 2.93 in the second week and 7.14 ± 2.56 in the fourth week. The frequency of defecations in the PEG group changed from 2.64 ± 2.12 per week before enrollment to 6.76 ± 3.07 in the second week and 6.74 ± 2.21 in the fourth week. These results demonstrated that although there was a significant improvement in both groups (P < 0.001), the defecation frequency was higher before treatment in the Jujube group than in the PEG group and increased after treatment.
Stool stiffness, scored according to the VAS, in the Jujube extract group changed from 87.14 ± 10.8 times per week before enrollment to 41.02 ± 24.34 in the second week and 27.35 ± 19.01 in the fourth week. Stool stiffness in the PEG group changed from 82.58 ± 13.3 per week before enrollment to 46.62 ± 22.23 in the second week and 38.38 ± 14.95 in the fourth week. These results demonstrated that although there was a significant improvement in both groups (P < 0.001), stool stiffness was higher in the Jujube group before treatment, which decreased significantly compared to the PEG group after two and four weeks of treatment (P-value < 0.0001).
The pain severity during defecation, according to the VAS, in the Jujube group changed from 67.14 ± 37.53 per week to 17.35 ± 27.14 in the second week and 12.85 ± 20.41 in the fourth week. The pain intensity in the PEG group changed from 71.29 ± 33.44 before treatment to 24.19 ± 31.75 in the second week and 17.26 ± 24.03 in the fourth week. Although there was a significant improvement in both groups, pain intensity was statistically significantly better in the Jujube group than in the PEG group (P < 0.001).
The frequency of stool withholding in the Jujube group changed from 8.16 ± 6.14 per week before the study to 1.86 ± 3.55 in the second week and 1.08 ± 2.42 in the fourth week, while it changed from 8.67 ± 5.3 before inclusion to 2.39 ± 4.39 in the second week and 1.56 ± 3.35 in the fourth week in the PEG group. These results show that although there was a significant improvement in both groups (P < 0.001), the frequency of stool withholding was significantly lower in the Jujube group than in the PEG group.
The frequency of large-diameter stools per week in the Jujube group changed from 2.69 ± 2.19 times per week before inclusion to 0.75 ± 1.59 times in the second week and 0.24 ± 0.78 times at the end of the fourth week. The frequency of large-diameter stools in the PEG group decreased from 2.84 ± 3.04 times per week before enrollment to 0.9 ± 1.5 times in the second week and 0.66 ± 0.94 times in the fourth week. These findings demonstrated a significant reduction in the frequency of large-diameter stools per week in both groups (P < 0.05).
The frequency of soiling per week changed from 2.86 ± 5.39 before the study to 1.53 ± 3.37 in the second week and 1.18 ± 2.56 in the fourth week in the Jujube group, while it changed from 1.68 ± 2.47 to 0.74 ± 2.62 in the second week and 0.64 ± 2.19 in the fourth week in the PEG group. These results demonstrated that although there was a meaningful improvement in both groups (P < 0.001), soiling decreased more in the Jujube group than in the PEG group.
Accordingly, the numbers of bowel movements, stool stiffness, painful defecation, stool withholding, and large-diameter stools in both Jujube and PEG groups significantly changed in the second and fourth weeks of treatment compared to before.
The frequency of soiling in the Jujube extract group significantly changed in the fourth week compared to before. And also, in the PEG group, soiling frequency significantly decreased in the second and fourth weeks compared to the beginning of treatment.
The frequency of bowel movements per week increased in both the Jujube and PEG groups. Also, the frequency of stool stiffness, painful defecation, withholding, large-diameter stools, and soiling per week decreased in both Jujube extract and PEG groups.
Regarding the side effects of the drugs, in the PEG group, 3.2% had mild abdominal pain, 6.5% had diarrhea, 9.7% had large stools that worried the child, and 80.6% did not report any side effects. In the Jujube group, 10.2% had mild abdominal pain, 4.1% had diarrhea, and 85.7% reported no side effects. The mean drug acceptance was 2.24 and 1.57 out of 7 in the PEG and Jujube groups, respectively.
5. Discussion
The mean age of the pediatric patients enrolled in our study was 5.3 ± 2.9 years in the Jujube group, 4.7 ± 2.6 years in the PEG group, and 4.99 ± 2.7 years in total. There was no significant difference between the two groups in age.
Among the seven criteria of Rome IV for FC, stool stiffness improved significantly by Jujube compared to PEG, but in other criteria, they had similar effects. It appears that improvement in defecation status in the Jujube group is mainly due to mucilage and fiber in Jujube. Water-soluble carbohydrate concentrates of jujube fruit containing glucose, fructose, pectin polysaccharide, and hemicellulose improve intestinal activity.
In 2009, Naftali et al. investigated the efficacy and safety of Jujube extract compared to a placebo in 37 adult patients with chronic constipation over 12 weeks. They assessed gastrointestinal transit time, the severity of symptoms, and quality of life before and after receiving liquid Jujube or placebo and found improvement in all parameters evaluated. Similar to our work, they concluded that Jujube is effective and safe in the treatment of chronic constipation; but unlike our study, they studied an adult group of patients nor children; they used different scales to evaluate the improvement of constipation; they used an extract of Jujube nor its paste; and the sample size was smaller than in our study (19).
Nasri et al., in a study performed in 2019, compared PEG with LaxaPlus Barij® Syrup in treating chronic constipation in 60 children aged 2 to 15 years. LaxaPlus Barij® Syrup is an herbal medicine prepared from the extract of Jujube, rose, asparagus, violet flower, borage, quince seeds, and Cordia myxa fruit. Their study results demonstrated that after eight weeks of intervention, LaxaPlus Barij®, compared to PEG, was more effective in the frequency of bowel movements and abdominal pain intensity (20). Compared to our study, they used a mixture of several herbal drugs, including Jujube, and improvement in constipation may not be attributed to Jujube alone.
In 2016, Esmaeilidooki et al. compared PEG and Floss (a traditional herbal drug) in treating children with FC. They studied 109 patients and concluded that Floss could have a therapeutic effect on FC in children compared to PEG. Similar to our study, children were treated with these two drugs for four weeks, and all Rome III criteria were improved in both groups (21). Floss, contrary to Jujube, has no nutritional value; also, Floss could not be used as a long-lasting drug due to intestinal irritation.
In 2015, Nimrouzi et al. compared the effects of PEG and sorrel plants on FC in children. This trial included 120 children with FC aged 2 - 12 years. Finally, both groups showed improvement in all criteria based on Rome III (22). Consumption of sorrel is more difficult than Jujube and has no nutritional value. Also, Jujube paste is more accepted by children and easier to use because it is sweet and can be mixed with food before giving it to children.
To evaluate the effect of fig syrup on constipation, studies were conducted in 2010 by Kim et al. and Baek et al. in 2016 (23, 24). Fig syrup showed a significant effect on constipation treatment. Although fig syrup is a good laxative, figs are more expensive than Jujube, and fig sugar base reduces its benefits versus Jujube paste.
Some studies also suggest belly massage with oils such as olive oil to relieve constipation in children (25-28). This method is good for relieving constipation but is not recommended for a long time for chronic constipation.
In our study, the incidence of complications was 14.3% in the Jujube group and 19.4% in the PEG group (P = 0.65), showing that the complication rate was not significantly different between the two groups. Neither group had a significant complication leading to discontinuation of the drug. Jujube causes milder colic pain than PEG; the Jujube group did not report voluminous stools, and diarrhea rarely occurred in both groups.
The acceptance rate of Jujube paste was higher than that of PEG in children, although, in both groups, the acceptance rate was in the desired range. Children probably accept Jujube better because of its good and dainty sweet taste. Jujube paste can even dissolve in water, syrup, or baby soup.
In this clinical trial study, the primary outcome was functional constipation maintenance treatment in children using Jujube paste compared to the PEG drug. The secondary outcomes were promoting the nutritional status and increased quality of life and growth status in FC children.
Limitations: In this study, like many similar studies, blinding was not possible because the two drugs were completely different in appearance, packaging, taste, and method of administration. A third person outside the treatment team evaluated the results. Following the patients for a longer treatment period is suggested in future studies.
5.1. Conclusions
In all the seven criteria of Rome IV, Jujube paste was as effective as PEG and even stronger in reducing stool stiffness. On the other hand, Jujube paste contains micronutrients and antioxidants, has nutritional properties compared with PEG, has a pleasant taste, is more accepted by children, and its consumption has no special side effects. Therefore, the authors suggest the Jujube paste for treating FC in children.