1. Background
Coronaviruses mainly cause mild-to-moderate respiratory disease like common cold. Since 2002, coronaviruses have caused two highly fatal epidemics and the recent COVID-19 pandemics, and produced catastrophic global effects (1). By the end of October 2022, more than 625 million cases were infected with SARS-CoV-2 infection, and more than 6.5 million people lost their lives (2).
SARS-CoV-2 has wide clinical presentations ranging from mild upper respiratory tract infection to severe respiratory distress syndrome (1). Pregnant women with SARS-CoV-2 infection face higher risks of severe disease as well as mortality and morbidity (3). SARS-CoV-2 infection during pregnancy ends with adverse neonatal outcomes proportional to the maternal disease severity. The severe maternal disease increases the prematurity rates and postnatal resuscitation need, and prolongs the hospital stays (4).
SARS-CoV-2 RNA is usually isolated from the respiratory tract swabs by Real-time Reverse-Transcription Polymerase Chain Reaction (RT-qPCR) test. RT-qPCR assay amplifies viral RNA up to a certain number of cycles, and the number of cycles exceeding the threshold reflects detectable viral load. Cycle threshold (Ct) value is the number of cycles required to cross the threshold, and the lower Ct value is suggestive of the higher viral load (5). The higher viral load of SARS-CoV-2 has been found associated with more severe disease course with lower lymphocytes and higher inflammatory biomarker levels (6). There are several discrepancies regarding the positive correlation between Ct values and disease severity in SARS-CoV-2 (7).
2. Objectives
This study aimed to examine the association between maternal viral load and maternal disease severity, as well as to prove the possible association between maternal viral load and neonatal outcomes.
3. Methods
This study was conducted between October 2020 and September 2021 at a single tertiary centre in Istanbul Basaksehir Cam and Sakura City Hospital, Turkey, after receiving the approval from local ethics committee (2021-220). Parental informed consent was also obtained prior to study. During the study period, pregnant women with one of the symptoms such as fever, cough, sore throat, myalgia, anosmia, diarrhea, and respiratory difficulty were tested by RT-qPCR test. All pregnant women with a positive SARS-CoV-2 RT-qPCR were included in the study. SARS-CoV-2 RNA was detected using the SARS-CoV-2 Double Gene RT-qPCR kit (Bio-speedy, Bioeksen, Istanbul, Turkey) on the Bio-Rad CFX96 Touch instrument (Hercules, CA, USA) in the Department of Medical Virology. Ct value was considered to be positive for SARS-CoV-2 if it was below 38. RT-qPCR test results and Ct values were obtained retrospectively from the patient records in the Virology Department.
Demographical and clinical data about 55 SARS-CoV-2 infected pregnant women and their newborn infants were assessed. Neonatal respiratory, hemodynamical, neurological and gastrointestinal morbidities and mortality were recorded. The association of Ct values with maternal SARS-CoV-2 infection disease severity as well as neonatal clinical and laboratory outcomes were examined.
The diagnosis of respiratory distress syndrome (RDS) was established based on the RDS guidelines of Turkish Neonatal Society (TNS). Bronchopulmonary dysplasia (BPD) was defined as ongoing oxygen need for first 28 days of life and at 36 weeks’ postmenstrual age. The Volpe classification was used for intraventricular haemorrhage (IVH) staging. Necrotising enterocolitis (NEC) diagnosis was made based on the clinical and laboratory findings defined in modified Bell’s criteria. ROP was staged as it was reported by the International Classification of Retinopathy of Prematurity.
Maternal disease severity was grouped as mild, moderate, or severe according to the symptoms and radiological findings. Mothers with only fever, cough, or myalgia were defined as mildly ill, and mothers having additional lung involvement were defined as moderately ill; mothers were defined as severely ill if they had respiratory difficulty, hemodynamical instability, and multiple organ dysfunction.
IBM SPSS 22.0 (IBM SPSS for Windows version 22, Armonk, NY) was used to perform statistical tests. Shapiro–Wilk test was performed to evaluate the normal distribution of variables. Mean±standard deviation was used for indicating descriptive statistics, median (minimum–maximum) for continuous variables, and n (%) for categorical variables. One-way ANOVA and Tukey’s HSD post hoc analysis tests were used to compare mean values. Fischer’s exact tests compared the categorical values, and P-value less than 0.05 was considered significant. Pearson’s correlation analysis test was also performed to examine the correlation.
4. Results
In this retrospective cohort study, a total of 55 pregnant women were tested by RT-qPCR test for SARS-CoV-2 and analyzed for Ct values along with their newborn infants.
The median age of pregnant women was 27 (16-40) years. Main delivery route was cesarean section (74.5%). The median Ct value was 30 (20 - 37), the median gestational age of infants was 38 (29 - 41) weeks, and the median birth weight was 3200 (630-4570) grams. Prematurity rate was 30% (n = 17). The demographical data of pregnant women are shown in Table 1.
| Disease Severity | Mildly Ill (n = 31) | Moderately Ill (n = 4) | Severely Ill (n = 20) |
|---|---|---|---|
| Maternal age, y | 26.5 (21 - 40) | 24 (16 - 35) | 31 (24 - 39) * |
| Gestational week, w | 39 (34 - 41) | 38 (36 - 39) | 36 (29 - 39) * |
| SARS-CoV-2 RT-PCR Ct value | 30 (20 - 37) | 28.5 (20 - 31) | 30 (21 - 36) |
| Delivery mode-Cesarean section | 22 (71) | 3 (75) | 20 (100) ** |
| Preterm delivery | 2 (6.4) | 1 (25) ** | 13 (65) ** |
| Hospital stay, d | 2 (2 - 15) | 10 (6 - 14) * | 15.5 (2 - 55) * |
| Mortality | 0 | 0 | 4 (20) ** |
Demographical Data of Pregnant Women Infected with SARS-CoV-2 a
There was no significant difference between mild, moderate or severely ill pregnant women in terms of median Ct values, (30, 28.5, and 30, respectively; P > 0.05). Preterm delivery and cesarean section rates were higher in severely ill pregnant women. The median Ct value of the pregnant women who gave preterm delivery was similar to that of those who had term delivery [30 (21 - 36) vs. 30 (25 - 35); P > 0.05]. Four severely ill pregnant women died, and overall mortality rate was 7.2%. The median Ct values of the pregnant women who died were similar to those of women who survived [32.5 (30 - 34) vs. 30 (21 - 36); P > 0.05]. Preterm delivery rates and overall mortality were also found not to be associated with SARS-CoV-2 RT-qPCR Ct values, (r = 0.04, P > 0.05). Ct values were not correlated with disease severity (r = 0.03, P > 0.05). The hospital stay of pregnant women was not correlated to Ct values, (r = 0.04, P > 0.05).
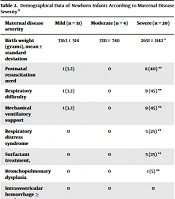
Nine (16.3%) newborn infants needed neonatal resuscitation, and 10 (18.1%) ones developed respiratory difficulty after birth. The need for postnatal resuscitation and mechanical ventilatory support was significantly higher in infants of mothers who were severely ill. RDS, Patent ductus arteriosus (PDA), and BPD were higher in premature infants of severely ill mothers. The hospital stay was similar for all groups, and there was no neonatal mortality. Demographical data of neonates are summarized in Table 2 based on the disease severity of mother. No association was detected between Ct values of mother and neonatal outcomes. (r = 0.1, P > 0.05).
| Maternal Disease Severity | Mild (n = 31) | Moderate (n = 4) | Severe (n = 20) |
|---|---|---|---|
| Birth weight (grams), mean ± standard deviation | 3363 ± 514 | 3311 ± 740 | 2651 ± 1142 * |
| Postnatal resuscitation need | 1 (3.2) | 0 | 8 (40) ** |
| Respiratory difficulty | 1 (3.2) | 0 | 9 (45) ** |
| Mechanical ventilatory support | 1 (3.2) | 0 | 9 (45) ** |
| Respiratory distress syndrome | 0 | 0 | 5 (25) ** |
| Surfactant treatment, | 0 | 0 | 5 (25) ** |
| Bronchopulmonary dysplasia | 0 | 0 | 1 (5) ** |
| Intraventricular hemorrhage ≥ grade 3 | 0 | 0 | 0 |
| Patent ductus arteriosus | 0 | 0 | 2 (10) ** |
| Necrotising enterocolitis | 0 | 0 | 0 |
| Retinopathy of prematurity | 0 | 0 | 0 |
| Hospital stay (days), median (min - max) | 2 (2 - 7) | 2 (1 - 2) | 2 (2 - 41) |
| Mortality | 0 | 0 | 0 |
Demographical Data of Newborn Infants According to Maternal Disease Severity a
5. Discussion
This retrospective study showed that maternal SARS-CoV-2 viral load indicated by Ct values was not associated with maternal disease severity and maternal/neonatal outcomes. The viral load was also found to have no effect on preterm delivery rates. In addition, total duration of maternal/ neonatal hospitalization and maternal/neonatal mortality were not associated with the viral load.
Defining the viral load is important when assessing the transmission potential of the virus. Even though the live virus has a relatively short life, SARS-CoV-2 RNA can be shed for longer periods. SARS-CoV-2 can be detected by RT-qPCR through amplifying viral RNA up to a certain number of cycles. The number of replicating cycles to exceed the threshold is defined as Ct. A low Ct is typically associated with high infectivity risk (5).
The association between viral load of SARS-CoV-2 and disease severity is controversial. Several studies have reported the correlation between viral load and disease severity, whereas some studies have found no relation. Patients with higher viral load and lower Ct values were reported to have higher mortality in a study with large sample size in Brazil (8). The higher viral load was also found to be associated with increased risk of intubation and higher mortality (9). Severe cases were determined to have higher viral load and longer viral presence in China (10). Another study demonstrated that Ct values were lower during the disease course in deceased patients than in recovered patients (11).
Although many studies supported the hypothesis of correlation between viral load and disease severity, there is a large body of studies from US with 5830 patients suggesting that Ct values were similar both in symptomatic and asymptomatic patients (12). Mean viral loads among patients with or without pneumonia were also found to be similar in another study (13). In our study, no association was detected between maternal viral load with the severity of disease.
There are several studies on vertical transmission and neonatal outcomes of SARS-CoV-2 infection, but available data about vertical transmission are conflicting. An original study carried out in Spain reported RT-PCR test positivity rates of 3% for infants of mothers with SARS-CoV-2 (14). Different systematic reviews reported RT-PCR test positivity of 1.9% and 4.2% for infants of SARS-CoV-2 infected mothers (15, 16). However, many studies showed no vertical transmission while RT-PCR tests were negative for neonates. Salvatore et al. (17) investigated 120 neonates of SARS-CoV-2 infected women and revealed that none of the infants was positive for SARS-CoV-2. Similarly, another review found no evidence of intrauterine or transplacental transmission of SARS-CoV-2 (18). Edlow et al. (19) attempted to quantitate SARS-CoV-2 viral load in maternal and neonatal biological fluids, and found no detectable viremia in maternal or cord blood as well as no SARS-CoV-2 RNA at placenta and, therefore, concluded that infection in placenta or vertical transmission of SARS-CoV-2 was not probable (19).
The Ct value has been suggested to function as a prognostic marker in viral infections. However, there are conflicting results about its efficacy in SARS-CoV-2 infections (20). No difference was observed between asymptomatic and symptomatic patients in terms of viral load (20). Ct values were also found strongly correlated with infectivity as lower the Ct values suggested higher infectivity (21). In a review including 18 studies, lower Ct values were reported to be associated with worse outcomes in COVID-19 patients (22). In a larger systematic review including 113 studies, SARS-CoV-2 viral loads were determined to be similar between symptomatic and asymptomatic patients (23). However, more data are required to establish the exact role of viral load in prediction of disease severity and prognosis in patients with SARS-CoV-2 infection.
In a cell culture model, a strong correlation was detected between Ct values and sample infectivity. It was shown that patients with Ct values ≥ 34 excreted no infectious virus particles and even had high viral load, and that the virus could not be isolated after day eight (21). Although Ct for infectivity was determined < 35 by two other studies, Ct values were not associated with clinical symptoms (24). As median and range levels were similar in our study, no cut off value was established for Ct.
Although contradictory data have been reported about the role of viral load in prediction and prognosis of SARS-CoV-2-infection, only few studies have explored the effect of viral load on maternal and neonatal outcomes. In studies conducted in India, no significant association was found between clinical symptoms and Ct levels of pregnant women infected with SARS-CoV-2 (24). Edlow et al. (19) quantified the viral load by copy counts/mL; viral load was not associated with any placental pathology. In contrast, one study from Turkey found a correlation between Ct values of pregnant women and perinatal/neonatal outcomes, and reported 22.9 for the 50th percent of Ct. Pregnant women with Ct values < 22.9 had poorer outcomes with higher obstetric complications, increased neonatal intensive care unit admissions, and prolonged duration of hospitalization in infants (25). Maternal Ct 50th percentile value was 30 in our study. No significant differences were detected among women with Ct values below or above 30 in terms of perinatal and neonatal outcomes. Similar to most studies in literature, therefore, Ct values was found not associated with maternal disease severity and also neonatal outcomes in our study.
Our small sample size and the lack of a control group were the main limitations of our study. Furthermore, none of the infants was positive for SARS-CoV-2 RT-PCR and, therefore, viral load of infants was not possible to evaluate, which was another important limitation of our study.
5.1. Conclusions
In sum, our study was one of the pioneering studies examining the association between maternal SARS-CoV-2 viral load and maternal/neonatal outcomes. It was revealed that the viral load of pregnant women may not have been used for predicting the severity of maternal disease and maternal/neonatal outcomes. However, it was recommended that further studies with larger sample sizes should be carried out to investigate the given issue.