1. Introduction
Febrile seizures are among the most common reasons for pediatric emergency visits. Seizures in early childhood are mostly associated with high fever (1). Febrile seizures are defined as seizures associated with a febrile illness that developed without central nervous system infection or acute electrolyte imbalance, intoxication, trauma, and metabolic disorder in children aged 1 month to 5 years without previous afebrile seizures (2-4). According to previous studies, the risk factors for febrile seizures were reported as male gender, family history of febrile seizures, high peak body temperature, anemia, and iron and zinc deficiencies (5-7).
Zinc is involved in the regular functioning of the immune system, growth and development, and enzymatic reactions in many organs. Zinc also plays a role in the body’s neurological functions, nerve conduction, and the regular release of hormones (8, 9). The role of zinc insufficiency in triggering febrile seizures has been revealed by some studies. Zinc modulates the level of gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter, via the pyridoxal kinase enzyme (10, 11).
2. Objectives
The purpose of this study was to investigate the role of serum zinc in the possible pathogenesis of children aged 6 months to 5 years admitted to the Pediatric Emergency Department of Prof. Dr. Cemil Taşcıoğlu City Hospital, Istanbul, Turkey, and diagnosed with febrile seizures.
3. Methods
3.1. Ethical Confirmation
This study was carried out with the permission given in the decision of the Ethics Committee of Prof. Dr. Cemil Taşcıoğlu City Hospital, Istanbul, Turkey, dated 27/09/2021, and numbered 337. The legal representative of each of the cases was included in the study and was enlightened about the study. Informed consent was obtained from those who agreed to be included in the study.
3.2. Study Plan
This prospective, cross-sectional, and descriptive study was conducted from 04/10/2021 to 04/02/2022. A total of 60 patients, including 30 patients between the age of 6 months and 5 years with fever and seizures and 30 patients with fever but not seizures, were included in the study as the patient group. The control group consisted of 25 patients in similar age groups admitted to the pediatric outpatient clinics for routine control and had no acute or chronic diseases. The body temperature of the patients included in the study was measured with a mercury thermometer in the armpit, and a temperature above 37.5°C was accepted as fever (12).
In patient groups, demographic characteristics, complete blood counts, biochemical parameters and serum zinc levels, fever on admission, type of seizures (complicated/simple), and other physical examination findings were recorded in detail. The number of complex seizures was very low (No.: 3), and it was not included in the study because it made comparisons difficult. As the control group, children in a similar age group with no seizures and fever, with normal neuromotor development and growth, and with no abnormality in the physical examination were included in the study. The exclusion criteria were the presence of cerebral palsy, electrolyte imbalance, central nervous system infection, chronic and/or genetic disease, zinc supplementation, metabolic disease, and antibiotic therapy.
3.3. Measurement of Zinc Levels
For the measurement of zinc levels, the blood sample taken was centrifuged at 4000 g for 10 minutes after waiting for 15 minutes, and the plasma was separated and stored in Eppendorf tubes in a deep freezer at -80°C until the study day. Zinc tests were performed with the PerkinElmer mass spectrometer device of Perkin Elmer company of USA origin working with inductively coupled plasma mass spectrometry (ICP-MS) technique.
3.4. Statistical Analysis
IBM SPSS 22 package software was used for the statistical examination of the data. Descriptive statistics for categorical variants were summarized as numbers and percentages, and statistics for continuous variables were summarized as mean and standard deviation. The chi-square test was used to check categorical variants between the two groups. The conformity of continuous variables to ordinary dispersion was tested with the Kolmogorov-Smirnov test of normality. Group comparison in normally distributed variables was made with a one-way analysis of variance (ANOVA), and then the Bonferroni test was used as a post-hoc test for various comparisons. The Kruskal-Wallis’ evaluation of variance was applied to the variables that did not show normal distribution, and pairwise comparisons were made with Dunn’s test. Pearson’s correlation analysis was performed for routinely distributed variables. Spearman’s correlation analysis was performed for the variables that did not show normal distribution. Cases with a P-value < 0.05 in all statistical tests were considered statistically significant.
4. Results
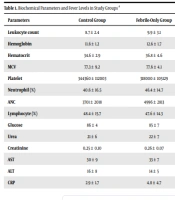
Of the 85 participants included in the study, 33 (38.8%) and 52 (61.2%) were female and male, respectively. The average age of the patients was 29.2 ± 15.9 months (range: 6 - 60). Of the 85 participating cases, 30 had febrile seizures, 30 were only febrile patients, and 25 were healthy controls. It was observed that gender distribution in the groups was statistically similar (P = 0.819). The median age was 28 (minimum: 6, maximum: 60), 32 (minimum: 8, maximum: 56), and 22 months (minimum: 8, maximum: 60) in control, fever-only, and febrile seizures groups, respectively, which was statistically similar (P = 0.320). Biochemical parameters and fever levels were examined in the study groups. Zinc level was lower in both only febrile and febrile seizure groups when checked against the control group (P = 0.041 and P = 0.008, respectively). Although the zinc levels in the febrile seizures group were lower than in the fever-only group, the distinction was not statistically significant (P > 0.05) (Table 1).
| Parameters | Control Group | Febrile-Only Group | Febrile Seizures Group | P-Value b |
|---|---|---|---|---|
| Leukocyte count | 8.7 ± 2.4 | 9.9 ± 3.1 | 13.7 ± 5.3 | < 0.001 c |
| Hemoglobin | 11.6 ± 1.2 | 12.6 ± 1.7 | 12.0 ± 1.5 | 0.051 c |
| Hematocrit | 34.6 ± 2.9 | 36.8 ± 4.6 | 36.1 ± 5.0 | 0.162 c |
| MCV | 77.3 ± 9.2 | 77.6 ± 4.1 | 77.7 ± 4.8 | 0.751 c |
| Platelet | 344360 ± 112003 | 318000 ± 105129 | 329467 ± 121054 | 0.691 d |
| Neutrophil (%) | 40.6 ± 16.5 | 46.4 ± 14.7 | 67.3 ± 12.6 | < 0.001 c |
| ANC | 3701 ± 2018 | 4996 ± 2813 | 8977 ± 4929 | < 0.001 c |
| Lymphocyte (%) | 48.4 ± 15.7 | 47.6 ± 14.3 | 25.1 ± 12.1 | < 0.001 c |
| Glucose | 86 ± 4 | 85 ± 7 | 101 ± 13 | < 0.001 c |
| Urea | 21 ± 6 | 22 ± 7 | 19 ± 6 | 0.179 c |
| Creatinine | 0.25 ± 0.10 | 0.26 ± 0.07 | 0.28 ± 0.12 | 0.872 c |
| AST | 30 ± 9 | 33 ± 7 | 35 ± 7 | 0.012 c |
| ALT | 16 ± 8 | 14 ± 5 | 14 ± 4 | 0.670 c |
| CRP | 2.9 ± 1.7 | 4.8 ± 4.7 | 13.0 ± 13.8 | 0.001 c |
| Sodium | 138 ± 2 | 138 ± 2 | 136 ± 2 | < 0.001 d |
| Potassium | 4.3 ± 0.4 | 4.5 ± 0.4 | 4.1 ± 0.5 | 0.004 d |
| Chloride | 101.8 ± 0.9 | 101.6 ± 1.9 | 100.8 ± 1.9 | 0.067 c |
| Zinc | 72.2 ± 8.6 | 66.1 ± 9.2 | 64.4 ± 11.9 | 0.024 c |
| Fever | 36.3 ± 0.1 | 38.3 ± 0.7 | 38.6 ± 0.9 | < 0.001 e |
Abbreviations: MCV, mean corpuscular volume; ANC, absolute neutrophil count; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CRP, C-reactive protein.
a Values are expressed as mean ± standard deviation.
b P < 0.05 is considered statistically significant.
c Kruskal-Wallis’ test
d One-way ANOVA test
e Chi-square test
In the febrile seizures group, some parameters, such as leukocyte count, absolute neutrophil count, neutrophil percentages, C-reactive protein (CRP), zinc levels, and fever values, were evaluated by correlation analysis. As a result of the analysis, no correlation was observed between these parameters (Table 2).
Abbreviation: CRP, C-reactive protein.
a Pearson correlation analysis was performed for these tests.
b The significance level is P < 0.05.
In the febrile-only group, there was a statistically significant positive correlation (P < 0.05) between CRP and leukocyte count and between neutrophil (%) and absolute neutrophil count. Moreover, there was a statistically significant negative correlation (P < 0.05) between zinc and leukocyte count (Table 3).
Abbreviation: CRP, C-reactive protein.
a Spearman’s correlation analysis was performed for these tests.
b The significance level is P < 0.05.
5. Discussion
In this study, zinc levels in patients with febrile seizures were measured, and it was shown that their plasma zinc levels were lower than in healthy controls. Some studies have reported that low zinc levels increase susceptibility to seizures. For example, it has been reported that changing dietary zinc intake can alter seizure susceptibility in an epileptic mouse model; low zinc levels increase susceptibility; high zinc levels have a protective effect. In addition, seizures were observed in rats who received intraperitoneal injections of sodium diethyldithiocarbamate, which is a zinc chelator (13). More importantly, zinc levels were observed to be remarkably lower in the blood and/or cerebrospinal fluid of children with febrile seizures than in both healthy controls and children presenting with fever only or non-fever-related seizures alone (14).
Mollah et al. compared serum and CSF zinc levels of children with febrile seizures to their peers without febrile seizures (15). The average serum and CSF zinc levels in children with febrile seizures were observed to be lower than in children who did not have seizures and fever (15). Bakri et al., in a case-control study, demonstrated that zinc concentrations were notably lower in children with febrile seizures than in children in the control group (16). When Ganesh et al. cross-checked serum zinc concentration in 38 simple febrile seizure cases with 38 controls of the same age, they obtained significant results between the two groups (17). Similar studies have been carried out in Turkey. In their study, Tutuncuoglu et al. examined serum and cerebrospinal fluid zinc levels in 35 patients, 15 with febrile seizures and 20 with fever but no seizures, and showed the serum zinc level of cases with febrile seizures to be significantly lower than the control group (18). Doğan et al. demonstrated that the serum zinc level of patients with febrile seizures was notably lower than the group with fever and no seizures (19). All these studies support the present study’s findings. Although numerous studies have shown the relationship between zinc deficiency and febrile seizures, Kumar et al. reported that zinc supplementation did not prevent these seizures in a study they conducted (20). There is a need for further studies on this subject, which also requires an explanation of the pathophysiology (20).
Significant amounts of free zinc in the brain have been observed to co-localize with glutamate in synaptic pouches. Zinc is condensed in glutamatergic pouches by the zinc transporter 3 (21). This zinc is discharged due to activity resulting in concentrations as high as 100 - 300 μM in the extracellular field (22). Synaptic zinc is mainly found in cortical and limbic structures (23). Multiple potential mechanisms are thought to underlie increased neuronal excitability under low synaptic zinc conditions; this is because extracellular zinc interconnects with a number of ion channel receptors (24, 25). Zinc inhibits a number of ionotropic receptors at synapses in the central nervous system, including GABAA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and N-methyl-D-aspartate (NMDA) receptors. The zinc blockade of GABAA receptors is involved in the underlying pathogenesis of seizures, and it is suggested that the blockade of GABAA receptors results in reduced neuronal network inhibition, triggering the seizure (21).
In the present study, the zinc levels of the febrile seizures group were similar to those of the group with fever only. However, zinc levels in these two groups were lower than in the healthy control group. Arul et al. compared the groups with and without febrile seizures and showed that the zinc level was significantly lower in the group with febrile seizures (26). Heydarian et al. also demonstrated lower zinc levels in children who had febrile seizures in a systemic review than those who did not have febrile seizures (27). The aforementioned data suggest that decreased zinc levels might be associated with not only seizures but also fever.
Children who have a febrile seizure often have an underlying illness that causes fever. Children with febrile seizures have higher body temperatures than children with fever only (28). In this study, fever levels were much high in the febrile seizures group, although there was no significant difference between the febrile seizures group and the febrile-only group. Zinc levels were similarly low, suggesting that zinc levels are more affected by the presence of fever. In addition, in the current study, a statistically significant negative correlation was observed between zinc and leukocyte count in the febrile seizures group. This correlation supports the relation of zinc with inflammation.
Previous studies showed that the neutrophil count is higher in children with febrile seizures than in children who do not have any seizures but have a fever (29). Teran et al. detected leukocytosis in 24% of patients and neutrophilia in 91% of patients with febrile seizures (30). Aydogan et al., in their study, found the frequency of leukocytosis to be 14.5% in children who were admitted to an emergency department due to febrile seizures for the first time (31). Mohebbi et al. demonstrated the leukocyte count to be 15,000 and above in patients with febrile seizures and suggested a relation between febrile seizures and leukocyte count (32). Ozturk et al. found that the leukocyte counts in patients with febrile seizures varied between 3,650 and 36,200, and in their study, the mean leukocyte value was observed to be 13,973; however, no significant relationship was noticed between this value and febrile seizures (33).
In the present study, leukocyte counts were observed at similar levels in the healthy control group and the group with fever but no seizures; nevertheless, it was found to be higher in the febrile seizures group than in both the control group and the group with only fever. This result, a higher leukocyte count in the febrile seizures group, was similar to those of previous studies. In the current study, although no correlation was observed between the leukocyte count and any parameter in the febrile seizures group, there was a statistically significant negative correlation between the leukocyte count and serum zinc levels in the febrile seizures group. A study by Someya et al. showed that zinc deficiency increases white blood cells in rats. This finding supports the findings of the current study (34). Similarly, in another study conducted on rats, zinc deficiency increased the total leukocyte count (35).
Febrile seizures in children mostly occur due to inflammatory fever. This inflammation causes the release of acute-phase reactants from the liver. C-reactive protein is among the most important acute-phase reactants. C-reactive protein reaches its highest values 24 - 48 hours after the inflammatory response. Studies have reported that children with febrile seizures have lower CRP levels than children without seizures but with high fever. The reason for this is suggested that the inflammatory response is slower in children with fever without seizures (28). Gontko-Romanowska et al. observed that CRP levels were notably lower in pediatric patients with febrile seizures than in febrile children without seizures (28). In different studies, it has been shown that CRP values reach higher levels in children with fever without seizures due to the slow progression of the inflammatory process (28, 29). Özkale et al. observed the CRP value of the control group to be higher than the febrile seizures group; however, the distinction was not statistically substantial (36).
In the present study, the CRP levels were observed to be similar between the control and febrile-only groups and higher in the febrile seizures group than in both the control and febrile-only groups. This increase in the CRP levels in the febrile seizures group in the current study might have been due to the decrease in hospital admissions during the coronavirus disease 2019 pandemic period, late admissions to the hospital, or an additional secondary bacterial infection from the late admissions.
The most important limitation of the present study is being single-centered with a small sample size. The scientific contribution will be greater by designing the study in larger patient groups and measuring zinc levels in both cerebrospinal fluid and blood.
5.1. Conclusions
In the current study, the patients with febrile seizures and fever had lower serum zinc levels than the control group. This zinc deficiency might have played a role in the etiopathogenesis of both febrile seizures and fever. Multicenter studies will elucidate the possible role of zinc.