1. Introduction
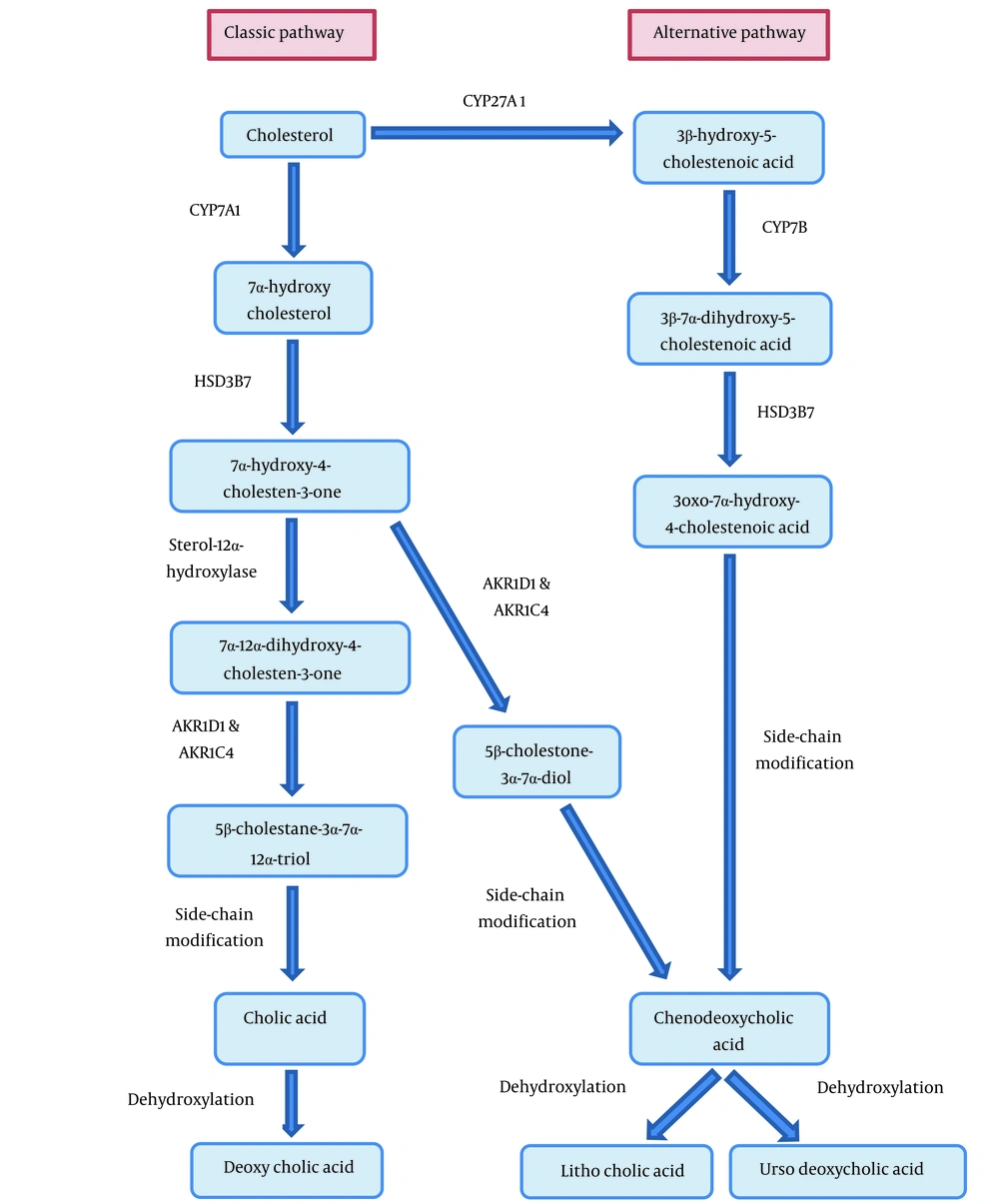
The bile acid synthesis disorder (BASD) is a very rare autosomal recessive disorder that is mainly diagnosed by detecting mutations in HSD3B7 (type 1) or AKR1D1 (type 2) genes, leading to deficiency in Δ4-3-oxosteroid-5β-reductase (Δ4-3-oxo-R) and 3β-Δ5-hydroxy-C27-steroid oxidoreductase (3β-HSD) enzymes, respectively (Table 1) (1, 2). The bile acid synthesis and metabolism are shown in Figure 1. These enzymatic impairments can result in the accumulation of atypical and hepatotoxic bile acid intermediates (3, 4). Clinically, these enzymatic defects can initially lead to cholestasis and progress to cirrhosis and hepatic failure, especially in untreated cases or in those with delayed diagnosis (5). More importantly, some specific symptoms and laboratory test changes of liver involvement, such as pruritus, increased γ-glutamyl transferase (GGT) activity, and raised serum bile acids are absent in BASD (6). Patients mostly suffer from failure to thrive (FTT) and symptoms of malabsorption in different organ systems, such as renal microcalculi and muscle weakness due to the malabsorption of fat-soluble vitamins (Vitamin E). The optimal diagnosis of BASD is based on molecular assessment with confirming genetic mutations as well as mass spectrometry analysis of urinary bile acids, indicating typical bile acid profiles (7). Early diagnosis of the disease is almost unlikely and the existing non-specific symptoms will confuse the clinical experts. BASD dramatically responds to bile acid replacement therapy such as cholic acid (CA) or chenodeoxycholic acid (CDCA). The most important effects of therapy are the down-regulation of bile acid synthesis and restoration of the bile flow (1). Herein, we described a case of BASD that was finally diagnosed using a genetic study after several years of workup.
| Enzyme Deficiency | Gene Encoding the Affected Enzyme |
|---|---|
| Cerebrotendinous xanthomatosis (sterol 27-hydroxylase deficiency) | CYP27A1 |
| Oxysterol 7α-hydroxylase deficiency | CYP7B |
| Cholesterol 7α-hydroxylase deficiency | CYP7A1 |
| 3β-hydroxy-Δ5-C27-steroid dehydrogenase deficiency | HSD3B7 |
| Δ4-3-oxosteroid-5β-reductase deficiency | AKR1D1(SRD5B1) |
| Alpha methylacyl-CoA racemase deficiency | AMACR |
| Zellweger syndrome (generalized peroxisomal β-oxidation disorders) | 12 PEX |
| Bile acid conjugation defects | BAAT and BAL |
2. Case Presentation
An Iranian 7-year-old girl was referred to us with FTT and multi-organ system involvement. She suffered from constipation from one to three years of age. Urinary tract infection with simultaneous renal microcalculi was detected at four years of age, and she has been under supervision and treatment by a pediatric nephrologist since then. Renal microcalculi were seen in serial sonographies with no significant changes in size and number despite treatment.
Muscle weakness appeared at five years of age; therefore, electromyography (EMG) and muscle biopsy were performed on the patient by a pediatric neurologist, and non-specific findings were reported. She has also suffered from nose bleeding since a year ago. She had consanguineous parents. Her cousin (a ten-year-old girl) had abdominal distention a year ago; thereafter, a liver biopsy was taken from her cousin, which revealed cirrhosis. The patient had FTT (weight: 17.5 kg, height: 112 cm). Laboratory assessment revealed impaired liver function tests (Aspartate Aminotransferase (AST): 96 IU/L (Normal reference range: up to 31 IU/L), Alanine Aminotransferase (ALT): 76 IU/L (Normal reference range: up to 31 IU/L), and Alkaline Phosphatase (ALP): 720 IU/L (Normal reference range: 180 - 1200 IU/L), γ-GGT: 20 (< 32 IU/L) and impaired coagulation tests (Prothrombin Time (PT): 17.5 seconds (Normal reference range: 11 - 13.5 seconds)). Some other important laboratory tests were as follows: albumin: 4.8 g/dL (Normal reference range: 3.5 - 5.2 g/dL) and tissue transglutaminase IgA: 88 U/mL (Normal reference range: < 20 U/mL). The patient underwent upper endoscopies three times with doubt of celiac disease. The pathological investigation reported mild chronic gastritis with no Helicobacter pylori and mild chronic duodenitis. No evidence of celiac disease was seen in the duodenal biopsies. Abdominal sonography showed normal liver with a hyperechoic lesion suggesting hemangioma, and the following computerized tomography (CT) scan confirmed the hemangioma. In this regard, and with the aim of discovering probable genetic abnormalities, a molecular assessment was recommended. Since the fast atom bombardment mass spectrometry (FAB-MS) technique was not used routinely in Iran and was not standardized for screening the profile of bile acids, we had to use a genetic test. In genetic exome sequencing, a homozygous pathogenic variant was identified in the HSD3B7 gene, consistent with the diagnosis of congenital BASD type I as an autosomal recessive disorder. The patient had to be treated with oral CDCA or CA; however, because of the unavailability of the drugs, she was treated with ursodeoxycholic acid, 250 mg, which led to growth compensation (weight: 22.5 kg, Height: 122 cm) and liver enzyme reduction after six months. The patient did not have any complaints from the presumable side effects of the treatment.
3. Discussion
Epidemiologically, the overall prevalence of BASD is estimated to be one per nine million people and consists of one to two percent of cases with unexplained liver diseases (8). The disease may occur at any age. The main causes of BASD include gene mutations that commonly encode enzymes involved in hepatic bile acid metabolisms, such as steroid oxidoreductase, cholesterol 7-alpha-hydroxylase, 2-methylacyl-CoA racemase, THCA CoA oxidase, and bile acid CoA ligase (1, 2). The deficiency in some of these enzymes, such as cholesterol 7-alpha-hydroxylase, may result in hypercholesterolemia without any evidence of liver cholestasis (2). In other words, the enzymes, which may be deficient in BASD, are encoded by the genes involved in bile acid synthesis and cholesterol metabolism. Clinically, cholestasis is a common finding in childhood; however, neurological manifestations are frequent in adults. Also, vitamin deficiency-related complications, such as rickets, neural dystrophy, bleeding diathesis, and night blindness, can be commonly seen (9). Overall, because of the unusual nature of the disease, delay in the diagnosis is very common. Common cholestatic liver diseases are associated with raised serum bile acid concentrations or γ- GGT levels, but the level of these markers is normal or low in BASD. Moreover, chronic cholestasis is commonly manifested by pruritus, which is absent in BASD. Due to the etiological genetic basis, the diagnosis of BASD can be achieved by mass spectrometry or confirming target gene mutation analysis, particularly by exome sequencing techniques. The assessment of serum hepatic enzymes and bilirubin profile may also help find differential diagnoses. Despite its ambiguous nature, BASD can be successfully treated with an excellent prognosis by CA or CDCA therapy (1).
Haas et al. assessed 36 patients with abnormal bile acid metabolites in their urine, of whom two patients had a diagnosis of BASD and 19 patients had peroxisomal disorders (10). In a recent report by Liu et al. in China (11), their case was presented with jaundice early after birth, pale stool, hepatomegaly, coagulopathy, cochlear nerve damage, liver cirrhosis, growth retardation, raised bilirubin and transaminase, gallbladder contraction decrease in ultrasonography, and normal bile ducts in cholangiography. Genetic testing of their case demonstrated a homozygous mutation in the AKR1D1 gene, confirming congenital BASD type 2. In a screening study by Al-Hussaini et al. in 2017 in Saudi Arabia (12), of 450 children with cholestasis, 15 children suffered from BASD presenting with cholestasis, cirrhosis, hepatomegaly, rickets, and liver failure. In further assessment, the gene mutations related to a deficiency in Δ-3-oxosteroid 5β reductase and 3β-hydroxy-Δ-C27-steroid oxidoreductase dehydrogenase were found to be the main cause of the disease. In our case, constipation and renal microlithiasis were the early presenting features and later muscle weakness and FTT, were prominent manifestations, which were misleading and led to the diagnosis of more common disorders, such as celiac disease, but the final diagnosis was achieved by genetic testing and detection of HSD3B7 gene mutation. Thus, when these non-specific symptoms appear, particularly during infancy, we should consider the BASD in our differential diagnosis list, and genetic testing should be considered as the main diagnostic tool. To the best of our knowledge, this is the first reported case of BASD in Iran, which was proved by a genetic study.
3.1. Conclusions
BASD is a disorder with diverse features and involvement of various organs, such as kidneys, liver, and muscles. These patients are referred to different specialists, and treatment is chosen based on the complaint relating to that organ. The final diagnosis was achieved after three years of symptoms and several diagnostic procedures in our case, which could be minimized by considering the BASD diagnosis at the first stages.