1. Background
According to the definition of international Children's Continence Society (ICCS), nocturnal enuresis (NE) is defined as monosymptomatic nocturnal enuresis, non-monosymptomatic nocturnal enuresis (enuresis with other lower urinary tract symptoms), and enuresis with the urological condition. The most common nocturnal enuresis is primary monosymptomatic nocturnal enuresis (PMNE) (1-3). The different etiological causes are involved in the development and exacerbation of PMNE. In general, PMNE is a medley and unorganized condition caused by several etiological factors (4, 5).
Vitamin D receptors are present throughout the body in skeletal and smooth muscles and the bladder. Vitamin D contributes to different clinical disorders such as asthma, respiratory tract infection, insulin disturbance, autoimmune disorders and metabolic syndrome, fertility, urinary tract infection, overactive bladder syndrome, chronic kidney diseases, and pelvic floor muscle disorder (6). Nowadays, many studies have indicated that vitamin D may be associated with lower urinary tract symptoms (LUTS); however, the findings are contradictory.
Numerous causes have been suggested for PMNE; however, the role of vitamin D in the development and severity of PMNE has yet to be comprehensively investigated. There is controversy about the relationship between vitamin D with enuresis (7). Furthermore, few studies have examined the relationship between vitamin D with LUTS and urinary incontinence; however, they have not addressed PMNE specifically. The association between vitamin D and non-skeletal disorders, especially enuresis, is not well-known, and further studies are required to confirm the existing findings.
2. Objectives
This study aimed to investigate the relationship between different vitamin D levels with the development and severity of PMNE to delve into PMNE severity.
3. Methods
3.1. Study Design and Setting
This research was a case-control study conducted in two tertiary primary care hospitals, Tehran, Iran, from June 2015 to December 2021. The participants were selected using the convenience sampling method.
3.2. Participants
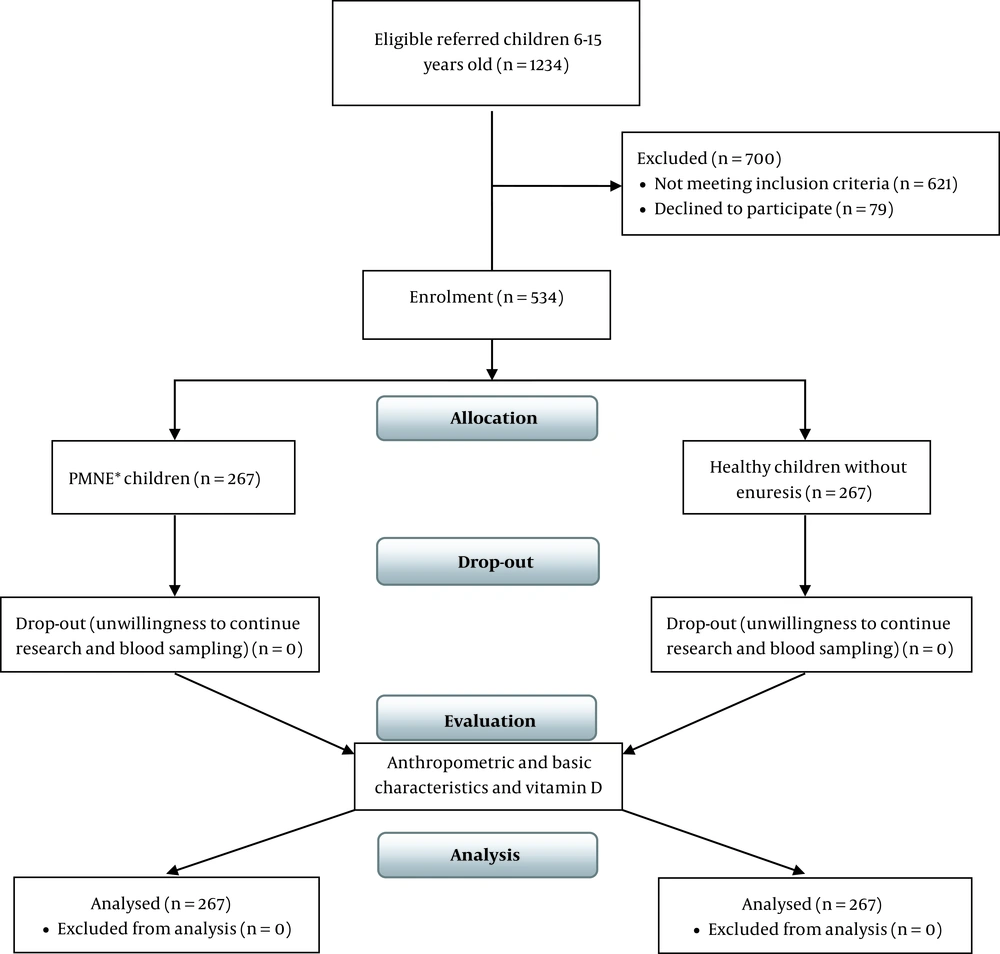
One thousand two-hundred thirty-four subjects aged 6 - 15 years referred to pediatrics- urology clinic were included in the study. Inclusion criteria were aged 6 - 15 years, ≥ 1 enuresis/week, > 3 weeks, only night time enuresis, never dry > 6 months (primary), and no urinary tract symptoms. Exclusion criteria were urological anomalies such as the ectopic ureter, neurogenic bladder dysfunction, spinal cord disorders, overactive bladder, urinary tract infection, obstructive sleep apnea, diabetics, polyuria, and vitamin D intake. Considering the following formula and reviewing previous studies,
and,
P0 = 25%, OR = 2, α = 5% and ß = 10%, 203 children were assigned to each group (N = 406). With a dropout of 20%, 488 children were included in the study. There were no dropouts due to the loss of follow-ups. Finally, 534 children aged 6 - 15 years old were enrolled in this study, and their data were analyzed. According to the ICCS definition (8), 267 children with PMNE were allocated to the case group. Considering the study criteria, 267 healthy children without enuresis who referred to the general pediatric clinic for check-ups and growth examination were selected as the control group (Figure 1). The severity of enuresis was defined as follows: Mild ≤ 2 times/week, moderate 3 - 4 times/week, and severe ≥ 5 times/week.
A comprehensive review of previous studies revealed the main confounders: Urological anomalies, neurogenic bladder, urinary tract infection, sleep disorders, snoring, diabetics, polyuria, vitamin D intake, liver disease, celiac, and anticonvulsant drugs. Non-basic and acquired variables were excluded by restricting attention to the inclusion and exclusion criteria. We concentrated on main and potential confounders in the PMNE children, which included age, gender, Body Mass Index (BMI), constipation, vitamin D status, income, parents’ education, and history of enuresis.
Constipation was evaluated according to ROME III criteria: Straining on feces, dry and hard feces, and feeling of imperfect defecation (at least two mentioned criteria) (9).
The confounders were controlled and minimized in three ways: Restricting the study subjects, matching the two comparison groups, and having pooled data stratifications. The restriction of the study subjects to two groups (children 6 - 15 years old) made the confounding factors be minimized. Furthermore, the other main and potential confounders were matched in two study groups. Regarding the effect modification, pooled data were stratified to stratum-specific variables, and stratified analyses were performed.
3.3. Vitamin D Assay
The serum samples were taken from all participants, centrifuged, and kept at -20°C. Vitamin D was assayed by ELISA kits (Pishtazteb Co., Tehran, Iran). Sensitivity, inter-assay, and intra-assay variation coefficients were 1.98 ng/mL, 15.8%, and 3.4%, respectively. According to the endocrine society, vitamin D levels were defined as follows: Deficiency 0 - 20 ng/mL, insufficiency 21 - 29 ng/mL, and sufficient 30 - 100 ng/mL (10).
3.4. Main Outcome Measures
The participants’ anthropometric parameters and basic characteristics, including such as gender, age, weight, height, BMI, constipation, vitamin D status, parents' education, and family history of enuresis were evaluated in both groups. Furthermore, the relationship between vitamin D levels with development and severity of enuresis was studied as the main outcome measure.
3.5. Statistical Analyses
Data analyses were performed with SPSS statistical software version 24 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean and standard deviations, and qualitative variables were described as frequency and frequency percentage. Differences between the two groups were determined using Student t-test and chi-square test. Furthermore, the Cochran-Mantel-Haenszel method was used forcontrolling for confoundingand statistical analysis was performed by multiple variable regression analysis to assay the odds ratio at a confidence interval of 95%. In this study, P < 0.05 were set as the significance level.
3.6. Ethics Statements
Informed consent was obtained from the participants' parents. During the research period, the names and information of parents and children were kept confidential. The Research Ethics Committee of the Aja University of Medical Sciences approved this study project (code: IR.AJAUMS.REC.1400.069).
4. Results
We evaluated 534 children, including 307 boys (57%), and 227 girls (43%). The mean age was 10.88 ± 2.28 years in the PMNE and 10.54 ± 2.29 years in the control groups. Anthropometrics measures in the PMNE and control groups were as follows: Weight: 57.22 ± 16.16 and 55.68 ± 16.82 kg; height: 146.29 ± 12.12 and 145.03 ± 12.28 cm; and BMI: 26.56 ± 5.71 and 26.09 ± 5.81 kg/m2, respectively (Table 1).
| Groups | PMNE | Control Group | P-Value | ||||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | ||
| Age (y) | 6 | 15 | 10.88 ± 2.28 | 6 | 15 | 10.54 ± 2.29 | 0.89 |
| Weight (kg) | 30 | 92 | 57.22 ± 16.16 | 30 | 88 | 55.68 ± 16.82 | 0.279 |
| Height (cm) | 123 | 166 | 146.29 ± 12.12 | 124 | 161 | 145.03 ± 12.28 | 0.235 |
| BMI (kg/m2) | 14.06 | 38.48 | 26.56 ± 5.71 | 11.89 | 35.4 | 26.09 ± 5.81 | 0.346 |
Abbreviations: PMNE, primary monosymptomatic nocturnal enuresis; BMI, body mass index; SD, standard deviation.
a The PMNE and healthy control groups were matched in terms of anthropometric measures.
All pooled basic and anthropometrics data were stratified to minimize confounders, and stratum-specific variables were analyzed. The crude and adjusted odds ratio (OR) at a confidence interval of 95% (95% CI) were shown for basic characteristics in the case and control groups (Table 2). The PMNE and control groups were homogenous in terms of anthropometric measures and basic characteristics (P > 0.05). However, there was a significant difference regarding the family history of enuresis between the two groups (P < 0.05). Adjusted ORs at 95% CI concerning the history of parental enuresis, father, mother, and both were 5.86 (95% CI 1.9 - 18.05, P = 0.002), 2.49 (95% CI 1.07 - 5.84, P = 0.035), and 15.03 (95% CI 1.88 - 19.6, P = 0.01), respectively. Furthermore, after adjusting for confounders, vitamin D was significantly associated with enuresis: Deficiency (< 20 ng/mL), OR 3.07 with 95% CI 1.9 - 4.95, P = 0.0001; insufficiency (20 - 30 ng/ mL), OR 2.72 with 95% CI 1.47 - 3.67, P = 0.0001). The parental history and the participants’ vitamin D status were significant risk factors for the PMNE development.
| Stratified Variables | PMNE, No. (%) | Control, No. (%) | Crude | Adjusted | P-Value |
|---|---|---|---|---|---|
| OR 95% CI (Lower–Upper) | OR 95% CI (Lower-Upper) | ||||
| Gender | 1.02 (0.72 - 1.43) | 0.97 (0.67 - 1.42) | 0.887 | ||
| Boy | 154 (57.7) | 153 (57.3) | |||
| Girl | 113 (42.3) | 114 (42.7) | |||
| Age, y | 1.26 (0.84 - 1.87) | 1.42 (0.82 - 2.45) | 0.21 | ||
| 6 - 9 | 67 (25.1) | 80 (30) | |||
| 10 - 12 | 142 (53.2) | 135 (50.5) | |||
| 13 - 15 | 58 (21.7) | 52 (19.5) | |||
| Height, cm | 1.35 (0.83 - 2.2) | 1.33 (0.66 - 2.67) | 0.421 | ||
| 123 - 130 | 36 (13.5) | 45 (19.5) | |||
| 131 - 140 | 57 (21.3) | 61 (22.8) | |||
| 141 - 176 | 174 (65.2) | 161 (60.3) | |||
| BMI, kg/m2 | 1.92 (0.16 - 22.1) | 1.95 (0.15 - 25.53) | 0.609 | ||
| Under w | 1 (0.4) | 2 (0.7) | |||
| Normal | 26 (9.7) | 27 (10.1) | |||
| Over w | 36 (13.5) | 30 (11.2) | |||
| Obese | 204 (76.4) | 208 (77.9) | |||
| Constipation | 0.89 (0.61 - 1.31) | 0.94 (0.62 - 1.41) | 0.774 | ||
| Yes | 68 (25.5) | 74 (27.7) | |||
| No | 199 (74.5) | 193 (72.3) | |||
| Level of education, father | 1.04 (0.71 - 1.53) | 1.06 (0.71 - 1.61) | 0.751 | ||
| < diploma | 85 (31.8) | 88 (33) | |||
| Diploma | 134 (50.2) | 133 (49.8) | |||
| > Diploma | 48 (18) | 46 (17.2) | |||
| Level of education, mother | 1.21 (0.85 - 1.72) | 1.19 (0.79 - 1.79) | 0.414 | ||
| < Diploma | 110 (41.2) | 123 (46) | |||
| Diploma | 133 (49.8) | 123 (46) | |||
| > Diploma | 24 (9) | 21 (8) | |||
| Parents’ enuresis history | 0.001 | ||||
| None | 217 (81.3) | 254 (95.1) | 1 | 1 | |
| Father | 19 (7.1) | 4 (1.5) | 5.53 (1.86 - 16.5) | 5.86 (1.9 - 18.05) | |
| Mother | 19 (7.1) | 9 (3.4) | 2.46 (1.09 - 5.53) | 2.49 (1.07 - 5.84) | |
| both | 12 (4.5) | 0 (0) | 13.99 (1.8 - 18.5) | 15.03 (1.88 - 19.6) | |
| Vitamin D, ng/mL | 0.0001 | ||||
| < 20 | 107 (40.1) | 57 (21.3) | 2.69 (1.72 - 4.22) | 3.07 (1.9 - 4.95) | |
| 20 – 30 | 109 (40.8) | 71 (26.6) | 2.32 (1.53 - 3.6) | 2.72 (1.47 - 3.67) | |
| > 30 | 51 (19.1) | 139 (52.1) | 1 | 1 |
Abbreviations: PMNE, primary monosymptomatic nocturnal enuresis; BMI, body mass index; W, weight.
a The PMNE and healthy control groups were matched in terms of anthropometrics measures and basic characteristics (P > 0.05). The participants’ parental history and vitamin D status were significant risk factors for the development of PMNE.
The prevalence of vitamin D deficiency and insufficiency was significantly higher in the PMNE groups (107 cases (40.1%) and 109 cases (40.8%), respectively) than in the control group (57 cases (21.3%) and 71 cases (26.6%), respectively) (P < 0.05).
Table 3 compared vitamin D levels between the PMNE and control groups. The mean and SD of vitamin D in the case and control groups were 18.58 ± 9.83 ng/mL and 30.23 ± 10.62 ng/mL, respectively. Vitamin D levels were significantly different between the two groups (P < 0.001). In other words, it vitamin D level was significantly lower in the PMNE group than in the control group.
| Vitamin D, ng/mL | PMNE | Control Group | |||
|---|---|---|---|---|---|
| No. (%) | Mean ± SD b | No. (%) | Mean ± SD | P-Value | |
| Total | 267 (100) | 18.58 ± 9.83 | 267 (100) | 30.23 ± 10.62 | 0.001 |
Abbreviations: PMNE, primary monosymptomatic nocturnal enuresis.
a In general, vitamin D level was significantly different between the two studied groups (P < 0.05).
b All values express by ng/mL
Table 4 presents the relationship between the severity of enuresis and vitamin D values. The vitamin D values in the PMNE children with severe, moderate, and mild enuresis were 10.6 ± 1.23 ng/mL, 19.46 ± 1.21 ng/mL, and 26.8 ± 2.61 ng/mL, respectively. The participants with a higher frequency of enuresis represented lower vitamin D levels (P = 0.0001).
| Severity of Enuresis | |||||||
|---|---|---|---|---|---|---|---|
| Vitamin D, ng/mL | Mild | Moderate | Severe | P-Value | |||
| No. (%) | Mean ± SD b | No. (%) | Mean ± SD | No. (%) | Mean ± SD | ||
| Total | 105 (39.3) | 26.8 ± 2.61 | 43 (16.1) | 19.46 ± 1.21 | 119 (44.6) | 10.6 ± 1.23 | 0.0001 |
Abbreviations: PMNE, primary monosymptomatic nocturnal enuresis.
a The participants with more frequency of enuresis seem to represent smaller vitamin D values (P = 0.0001).
b All values express by ng/mL
5. Discussion
The present study mainly aimed to evaluate the role of vitamin D levels in the development and severity of bedwetting in PMNE. This study defined that vitamin D levels were significantly lower in the PMNE children than in the control group. The prevalence of vitamin D deficiency and insufficiency was significantly higher in the enuretic participants. Furthermore, children with higher frequency of bedwetting represented lower vitamin D levels. There was a negative relationship between vitamin D levels and the severity of enuresis. That the participants’ parental history and vitamin D status were significant risk factors for the PMNE development.
Nocturnal enuresis is a universal public issue among boys and girls. The diverse etiological drawbacks are inducted in the development and severity of PMNE; however, the relationship between vitamin D with enuresis and other non-skeletal effects is still questionable and controversial (11-14).
The parental history of NE is a potential risk factor in the PMNE development. Most studies have revealed a positive parental history in about 36% of children. These children were 10.1 times more likely to have NE (15, 16). In our study, the prevalence of parental history was 18.7% and the odds ratio of enuresis was 15.03 times higher if both parents had a positive history of NE. This study showed that participants’ parental history and vitamin D status were significant risk factors for the PMNE development. Vitamin D deficiency may be inherited (17). The genetic evaluation of these families may help elucidate and identify a genetic abnormality. When identified, gene products could open up a new treatment modality for PMNE. To sum up, studying parents' genotypes, clinical symptoms for Vitamin D deficiency, and Vitamin D levels and acquiring the children's genotypes would be of significant genetic interest for future studies.
The frequency of constipation in children was between 0.7 - 29.6%. There are conflicting reports about of the relationship between constipation and enuresis. Some studies have indicated that constipation is a critical risk factor for LUTS; however, other studies revealed no significant relationship between constipation and enuresis (18). Sampaio et al (19) found out that the frequency of constipation in children with enuresis was 12%, which was not statistically significant. In our study, the frequencies of constipation in the control and case groups were 25.5% and 27.7%, respectively. However, there was no statistically significant difference between the two groups in this regard. Contradictory findings in this field are caused by different definitions of constipation in various studies. To reach precise and accurate findings, it is better to consider the standard definition of constipation (9).
A few studies have examined the relationships between vitamin D levels with LUTS and UI; however, they have not addressed PMNE specifically. A study on 50 PMNE Egyptian children showed that vitamin D values were smaller in healthy non-bedwetting children. Vitamin D < 20 ng/mL was observed in 23 subjects (46%) in the enuretic group and only 8 children (16%) in the control group (20). In this study, vitamin D levels were divided into two categories (< 20 and > 20 ng/mL), and the relationship between vitamin D and the severity of bedwetting was not studied. Our study included 534 children, and vitamin D levels were defined in three categories of deficiency, insufficiency, and sufficient. Furthermore, the relationship between vitamin D values and the severity of enuresis was also examined.
A systematic review suggested that an insufficient vitamin D level increased the likelihood of LUTS in the patients (1.37 - 2.06 times), and the patients with LUTS had lower vitamin D values (21). In this study, the relationship between vitamin D levels with NE and PMNE was not addressed, and the relationship between vitamin D with the severity of symptoms was not investigated. The present study indicated that vitamin D deficiency and insufficiency was significantly higher in the PMNE group (1.72 - 4.22 and 1.53 - 3.6 times, respectively). Furthermore, Children with more severe enuresis represented lower vitamin D levels.
A cross-sectional study on 247 NE children revealed that sufficient vitamin D was a protective agent for NE. Furthermore, they demonstrated that the frequency of enuresis was correlated with vitamin D levels (22). The findings of this study were similar to the present ones; however, they did not evaluated PMNE, and the participants were 5 - 7 years old.
The findings regarding the role of vitamin D in improving urinary symptoms are controversial. A few studies have investigated the effects of vitamin D on NE and overactive bladder. A study found that vitamin D supplementation could decrease the frequency of bedwetting in children (23). Another study showed that vitamin D deficiency was more prevalent in overactive bladder children, and that vitamin D intake may improve LUTS (24). In another study, however, vitamin D supplementation was not recommended for this purpose (25).
Several mechanisms have been expressed regarding the effects of vitamin D on bladder function. Vitamin D receptors are found in skeletal and smooth muscle cells throughout the body. Vitamin D receptors are present in both the detrusor muscle and the urothelium of the bladder (26). Vitamin D reduces bladder contractions by suppressing bladder sensory signals during the bladder filling phase. Vitamin D deficiency can increase uninhibited bladder contraction (27). Furthermore, cathelicidins play a key role in bladder mucosal immunity. Vitamin D facilitates the production of cathelicidins. A low level of vitamin D can increase the risk of recurrent urinary tract infection and subsequently, the prolonged presence of microbiomes in the bladder leads to bladder dysfunction (28). On the other hand, the low level of vitamin D increases the renal expression of endothelin-1 and decreases the epithelial sodium channel activity, which is probably responsible for natriuresis (29). In sum, the low level of vitamin D can lead to various pathological situations. The existing evidence confirms the relationship between vitamin D deficiency and NE; however, more precise and comprehensive studies are required to affirm these findings.
Behavioral intermediations are the first line of treatment in NE. Furthermore, drugs are useful and should be utilized in association with behavioral interventions. Such interventions must be initiated with determined reasons in mind. One of the reasons of treatment failure may be vitamin D deficiency (30), which may clarify and describe one of the reasons for the treatment break. The best strategy to employ proper treatment is using guidelines in cases of controversy in medicine. The guidelines can be assisting to string primary care practitioners views on precise treatment, as has been seen in the proper antimicrobials prescribing in different infectious diseases (31, 32).
One of the limitations of this study was that the effect of vitamin D supplementation on enuresis development was not evaluated. Clinical trials or cohort studies are recommended to be performed using vitamin D supplementation, and its effect on the severity of enuresis should be investigated to confirm these findings. Although it is not recommended to evaluate the vitamin D level for every child, it should be considered in cases resistant to treatment. It is recommended that all pediatricians should be informed of the enuresis status to select the foremost and excellent route to treat each child and consider the status of vitamin D when treating such patients.
5.1. Conclusions
According to the findings of this study, vitamin D levels were significantly lower in the PMNE children. The prevalence of vitamin D deficiency and insufficiency was significantly higher in the enuretic participants. Furthermore, children with higher frequency of bedwetting represented lower vitamin D levels. Furthermore, the participants’ parental history and vitamin D status were significant risk factors for the PMNE development. Regarding the vitamin D level and PMNE, preliminary evidence is accumulating; however, more comprehensive and precise studies are required to clarify the topics of uncertainty. This study provided the grounds to define clues for the reasons of PMNE and its severity.