1. Background
Human adenovirus (HAdV) is one of the most significant infectious agents affecting the respiratory system in children and is responsible for 5 - 10% of acute lower respiratory infections (RIs) in infants and children (1). HAdV infection is highly prevalent among infants aged 6 months to 2 years due to their lack of humoral immunity (2, 3). The severity of HAdV-induced pneumonia is much higher than that of ordinary pneumonia (4). Severe pediatric HAdV RI can lead to acute respiratory failure, chronic complications, and an increased risk of mortality (5). Lung sequelae are observed in approximately 14 - 60% of cases of HAdV-induced pneumonia (6). Severe HAdV pneumonia is a major contributor to patient deaths and disabilities among infants with pneumonia.
The incidence of severe HAdV RI increased significantly in Hunan in the winter and spring of 2019 compared to previous years. The role of HAdV in severe RI is increasingly recognized due to a marked rise in hospitalization and mortality rates (7). Although HAdV types, age of onset, and immunocompromised states have been reported as risk factors for severe adenoviral RI in children (2, 8, 9), the risk factors for children remain controversial. Determining the risk factors related to the severity of adenoviral RI is, therefore, a focus of current research.
The enzyme aspartate aminotransferase (AST) is expressed in various cell types, primarily in cardiomyocytes, followed by liver and kidney cells, with only a low concentration in normal serum. Cell damage results in increased permeability of the cell membrane, leading to the release of AST into the serum. The AST level has been shown to be associated with the severity of diseases such as acute pancreatitis, non-alcoholic fatty liver disease, and severe pneumonia (10-12). Children with HAdV infection often exhibit abnormal liver function. Tian et al. (13) demonstrated that the serum levels of AST or LDH correspond to the severity of adenoviral RI more closely than other indicators of liver injury during hospitalization. However, there have been no reports on the independent role and predictive value of AST as an indicator.
2. Objectives
Therefore, we conducted a retrospective review, analyzing the clinical data of 665 children with HAdV RI admitted to our hospital in 2019. We further evaluated the relationship between AST levels and the severity of the disease in children. It is hoped that this study will provide medical evidence for the early clinical diagnosis and targeted therapy of severe cases, thereby improving patient prognosis.
3. Methods
3.1. Subjects
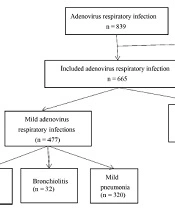
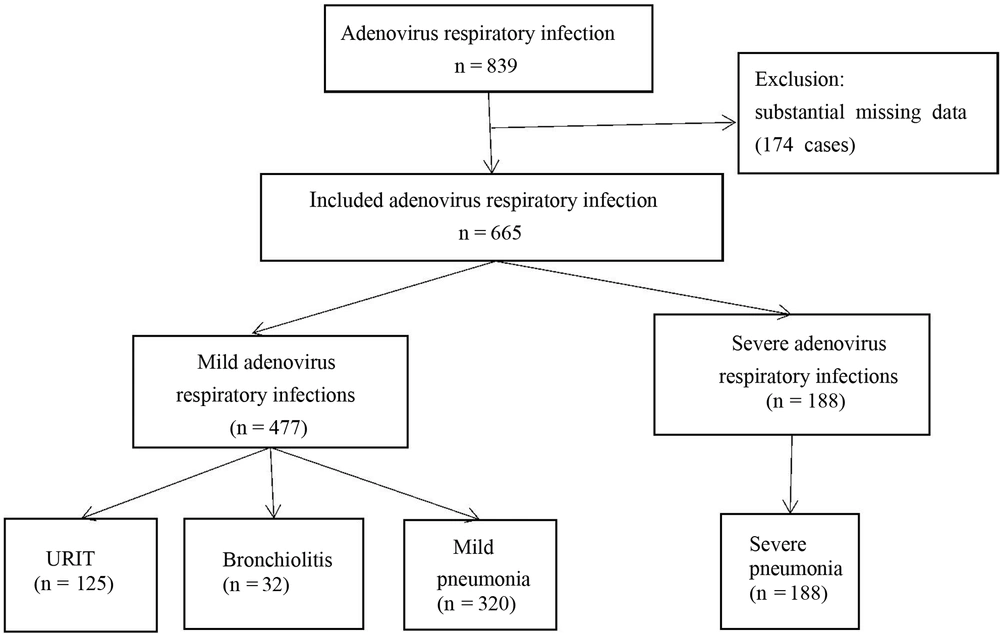
This is a retrospective investigation that included children aged < 14 years admitted to the pediatric ward of the First People's Hospital of Changde City due to HAdV RI from January 2019 to December 2019. During our investigation, all hospitalized children underwent standard testing for respiratory viruses. Finally, 665 cases of HAdV RI were deemed eligible for participation in this study (Figure 1).
3.2. Inclusion and Exclusion Criteria
The criteria included in the analysis were as follows: Children under 14 years old and the detection of HAdV in the patient's nasopharynx via RT-PCR analysis.
The criteria excluded from the analysis were as follows: HIV infection, malignancy, diagnosed or suspected tuberculosis, ongoing immunosuppression therapy, immunodeficiency, severe organ dysfunction, chronic illnesses, such as congenital heart or chronic lung disease, and cases with substantial missing data.
Clinical information, including symptom manifestations, routine examinations, and diagnostic data, was collected through the screening of medical records. Our study received approval from the review board at the First People's Hospital of Changde City. Informed consent was waived due to the retrospective design of our research.
3.3. Disease Severity
The 665 children who had HAdV-induced respiratory infections were divided into two groups based on their symptoms and signs: An upper respiratory infection (URI) group and a lower respiratory infection (LRI) group. The LRI group included cases of acute bronchiolitis and pneumonia. The severity of pneumonia was classified according to the WHO guidelines (14), and the diagnosis of severe community-acquired pneumonia (CAP) was made if one of the following symptoms was present: Central cyanosis, inability to nurse or drink, accompanied by vomiting, convulsions, lethargy, loss of consciousness, or severe respiratory distress.
All participants were categorized into one of two cohorts: Those with mild HAdV respiratory infections (URI, bronchiolitis, and mild pneumonia) or those with severe HAdV respiratory infections (severe pneumonia).
3.4. Sample Collection
Nasopharyngeal aspirates (NPAs) were collected from all participants within 24 hours of hospitalization, and the presence of HAdV was quantitatively assessed via polymerase chain reaction (PCR). Simultaneously, venous blood was collected for complete blood counts, C-reactive protein (CRP), myocardial enzyme, and liver function analyses.
3.5. Ethics Statement
The present study protocol was reviewed and approved by the Review Board of The First People's Hospital of Changde City (approval No. 2020-145-01). Informed consent was obtained from all subjects upon their enrollment.
3.6. Statistical Analysis
All analyses were conducted using SPSS 25.0 (IBM Corp., Armonk, NY, USA). Rate comparisons were performed with the χ2 test. Inter-group comparisons were made using the t-test for data with a normal distribution and the nonparametric Wilcoxon rank-sum test for data with an abnormal distribution. All variables that showed an independent association with HAdV RI in univariate analysis were further analyzed using multivariable analysis. The predictability of the risk factors in distinguishing between mild and severe cases of adenoviral respiratory infections was examined using a receiver operating characteristic (ROC) analysis and the area under the curve (AUC). A significance threshold of P < 0.05 was set for all analyses.
4. Results
The clinical demographics were examined to gather general information about the patients. Out of the 665 analyzed cases, 409 were male, and 256 were female, resulting in a male-to-female ratio of 1.69:1. The ages ranged from 1 month to 13 years, with a median age of 3 years (IQR: 1 - 4 years). Children under 6 years of age accounted for 89.8% of the cases.
HAdV-induced respiratory infections included upper respiratory infections (125 cases, 18.8%), acute bronchiolitis (32 cases, 4.8%), mild pneumonia (320 cases, 48.1%), and severe pneumonia (188 cases, 28.2%). Among them, 60 patients (9.0%) required oxygen, 68 cases (10.2%) needed mechanical ventilatory assistance, and 1 case (0.2%) resulted in death due to severe pneumonia. The median values of alanine aminotransferase (ALT), lactic acid dehydrogenase (LDH), creatine kinase (CK), creatine kinase isoenzyme (CK-MB), and AST concentrations were highest in the severe HAdV RI group. The median values of age, hemoglobin (HGB), CRP, and serum albumin (ALB) were lowest in the severe group (Table 1).
| Variables | URTI (n = 125) | Bronchiolitis (n = 32) | Mild Pneumonia (n = 320) | Sever Pneumonia (n = 188) | Total (n = 665) |
|---|---|---|---|---|---|
| Gender (male) | 65 (52) | 17 (53.10) | 204 (63.70) | 123 (65.40) | 409 (61.50) |
| Median age, y | 4 (0.08 - 13.75) | 4 (1 - 12.25) | 3 (0.08 - 12) | 1 (0.08 - 11) | 3 (0.08 - 13.75) |
| < 0.5 | 3 (2.40) | 0 (0) | 8 (2.50) | 23 (12.23) | 34 (5.11) |
| 0.5 - 2 | 32 (25.60) | 8 (25) | 126 (39.38) | 118 (62.77) | 284 (42.71) |
| 2 - 5 | 63 (50.40) | 20 (62.50) | 149 (46.56) | 40 (21.28) | 272 (40.90) |
| > 5 | 27 (21.60) | 4 (12.50) | 37 (11.56) | 7 (3.72) | 75 (11.28) |
| Median length of hospitalization, day | 4.95 (2 - 9) | 5.50 (4 - 8) | 6.55 (2 - 35) | 11.89 (3 - 34) | 7.71 (2 - 35) |
| Median WBC, 109/L | 7.56 (3.06 - 21.27) | 8.1 (3.32 - 25.74) | 7.27 (2.20 - 29.70) | 7.64 (0.82 - 35.19) | 7.44 (0.82 - 13.75) |
| Median HGB, g/L | 115.50 (77 - 170) | 112 (100 - 127) | 114.50 (64 - 142) | 108 (80 - 145) | 113 (64 - 120) |
| Median PLT, 109/L | 226 (109 - 524) | 250 (128 - 424) | 228.50 (84 - 690) | 231 (38 - 767) | 229 (38 - 767) |
| Median CRP, mg/L | 18.19 (0.20 - 145.50) | 15.79 (0.60 - 87) | 11.95 (0.20 - 242.90) | 11.6 (0.20 - 138.35) | 13.30 (0.2 - 242.90) |
| Median serum ALB, g/L | 39.65 (29.20 - 46.70) | 39.80 (34.90 - 45.10) | 39.60 (21.60 - 46.80) | 37.50 (25.70 -48) | 39.30 (21.60 - 48) |
| Median ALT, U/L | 13 (2 - 358) | 12 (7-195) | 14 (2 - 274) | 20 (6 - 259) | 15 (2 - 35.80) |
| Median AST, U/L | 32 (18 - 398) | 35 (20 - 120) | 39 (18 - 189) | 58 (23 - 770) | 41 (18 - 770) |
| Median CK, U/L | 150.79 (16 - 3805) | 152.63 (33,1438) | 136.06 (26 - 1663) | 247.90 (31 - 4206) | 171.58 (16 - 4206) |
| Median CK-MB, U/L | 25.02 (4.80 - 60) | 30.24 (13 - 99.90) | 26.98 (8.50 - 75.30) | 37.54 (13.40 - 135.50) | 29.80 (4.80 - 135.50) |
| Median LDH, U/L | 282.36 (177 - 623) | 309.28 (205 - 637) | 333.33 (169 - 847) | 678.12 (212 - 3247) | 421.31 (169 - 3247) |
Abbreviations: WBC, white blood cell; HGB, hemoglobin; PLT, platelets; CRP, c-reactive protein; ALB, albumin; ALT, Alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactic acid dehydrogenase; CK, creatine kinase; CK-MB, creatine kinase isoenzyme.
a Continuous variables are expressed as median (range) and categorical variables as No. (%)
In total, 665 patients were included in this investigation. Among them, 477 had mild adenoviral respiratory infections, and 188 had severe adenoviral respiratory infections (Table 2). Compared to children with mild adenovirus RI, those with severe adenovirus RI were more likely to have coinfections with mycoplasma, had longer hospital stays, and had lower age, HGB, ALB, and higher AST, ALT, CK, CK-MB, and LDH levels (P < 0.05).
| Variables | Mild Adenovirus Infection (n = 477) | Severe Adenovirus Infection (n = 188) | P-Value |
|---|---|---|---|
| Gender (male) | 286 (59.96) | 123 (65.43) | 0.192 |
| Age, y | 3.44 (0.08 - 13.75) | 1.78 (0.08 - 11) | 0.000 b |
| < 0.5 | 11 (32.35) | 23 (7.65) | |
| 0.5 - 2 | 166 (58.45) | 118 (41.55) | |
| 2 - 5 | 232 (85.29) | 40 (14.71) | |
| > 5 | 68 (90.67) | 7 (9.33) | |
| Hospital stay, d | 6.06 (2,35) | 11.89 (3,34) | 0.000 b |
| Breast feeding | 146 (30.61) | 55 (29.26) | 0.633 |
| Coinfection with mycoplasma | 24 (5.03) | 18 (9.57) | 0.030b |
| CRP, mg/L | 23.07 (0.20 - 242.90) | 21.35 (0.20 - 138.35) | 0.400 |
| WBC, 109/L | 8.43 (2.20 - 29.70) | 9.03 (0.82 - 35.19) | 0.440 |
| HGB, g/L | 114.36 (64 - 170) | 108.88 (80 - 145) | 0.000 b |
| PLT, 109/L | 250.40 (84 - 690) | 269.72 (38 - 767) | 0.757 |
| ALB, g/L | 39.67 (21.60 - 46.8) | 37.66 (25.70 - 48) | 0.000 b |
| AST, U/AL | 42.45 (18 - 398) | 78.69 (23 - 770) | 0.000 b |
| ALT, U/L | 19.06 (2 - 358) | 28.43 (6 - 259) | 0.000 b |
| CK, U/L | 141.01 (16 - 3805) | 247.90 (31 - 4206) | 0.000 b |
| CK-MB, U/L | 26.70 (4.80 - 99.90) | 37.54 (13.40 - 135.50) | 0.000 b |
| LDH, U/L | 318.47 (169 - 847) | 678.12 (212 - 3247) | 0.000 b |
Abbreviations: WBC, white blood cell; HGB, hemoglobin; PLT, platelets; CRP, c-reactive protein; ALB, albumin; ALT, Alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactic acid dehydrogenase; CK, creatine kinase; CK-MB, creatine kinase isoenzyme.
a Values are expressed as No. (%) or No. (range).
b P < 0.05.
To investigate the correlation between AST and HAdV RI severity, additional examinations were conducted. Univariable analysis revealed that age, HGB, PLT, ALB, ALT, AST, CK, CK-MB, and LDH exhibited the most significant inter-group differences. Individuals with severe HAdV RI showed lower onset age, HGB, and ALB while having higher PLT, ALT, CK, CK-MB, LDH, and AST levels (P < 0.05). The crude odds ratios for severe HAdV RI are presented in Table 3.
| Variables | Odds Ratio (95%CI) | P-Value |
|---|---|---|
| Age, year | 0.637 (0.569 - 0.714) | 0.000 a |
| Male gender | 1.264 (0.889 - 1.797) | 0.192 |
| WBC count, 109/L | 1.030 (0.993 - 1.068) | 0.118 |
| HGB, g/L | 0.957 (0.941 - 0.972) | 0.000a |
| PLT, 109/L | 1.001 (1.001 - 1.003) | 0.048a |
| CRP, mg/L | 0.990 (0.991 - 1.004) | 0.461 |
| Serum ALB, g/L | 0.840 (0.796 - 0.886) | 0.000a |
| ALT, U/L | 1.010 (1.004 - 1.017) | 0.002 a |
| AST, U/L | 1.032 (1.024 - 1.040) | 0.000 a |
| CK, U/L | 1.001 (1.001 - 1.002) | 0.001 a |
| CK-MB, U/L | 1.048 (1.035 - 1.062) | 0.000 a |
| LDH, U/L | 1.009 (1.007 - 1.011) | 0.000 a |
Abbreviations: WBC, white blood cell; HGB, hemoglobin; PLT, platelets; CRP, c-reactive protein; ALB, albumin; ALT, Alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactic acid dehydrogenase; CK, creatine kinase; CK-MB, creatine kinase isoenzyme.
a P < 0.05
Multivariable analysis was employed to confirm the relationships between the aforementioned variables and severe HAdV RI, with adjustments made for sex (Table 4). In the mild vs. severe group model, onset age, HGB, serum ALB, PLT, CK, CK-MB, LDH, ALT, and AST remained strongly associated with severe HAdV RI (P < 0.05).
| Variables | OR a (95%CI) | P-Value |
|---|---|---|
| Age, y | 0.639 (0.570 - 0.716) | 0.000 b |
| HGB, g/L | 0.957 (0.941 - 0.972) | 0.001 b |
| PLT count, 109/L | 1.001 (1.001 - 1.003) | 0.048 b |
| Serum ALB, g/L | 0.840 (0.796 - 0.886) | 0.001 b |
| ALT, UL | 1.010 (1.003 - 1.017) | 0.003 b |
| AST, UL | 1.032 (1.024 - 1.040) | 0.000 b |
| CK, UL | 1.001 (1.001 - 1.002) | 0.001 b |
| CK-MB, UL | 1.041 (1.027 - 1.055) | 0.000 b |
| LDH, UL | 1.008 (1.007 - 1.010) | 0.000 b |
Abbreviations: HGB: hemoglobin; PLT, platelets; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
a Adjusted for gender.
b P < 0.05.
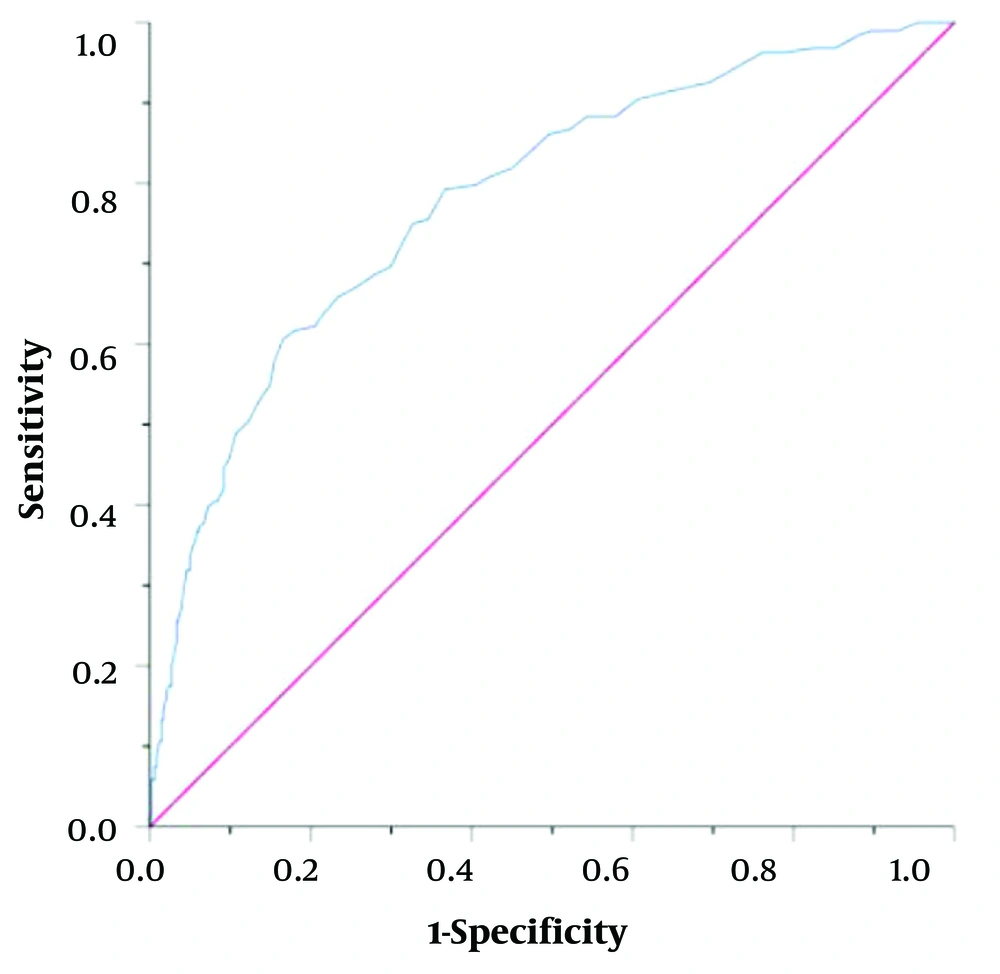
To assess the diagnostic efficacy of AST in evaluating disease severity, ROC curve analysis was conducted to evaluate the predictive value of AST as an indicator of disease severity. The area under the AST ROC curve was 0.782, with a sensitivity of 0.606 and a specificity of 0.834. The threshold for the serum AST level was 52.5 U/L for severe HAdV RI (Figure 2). These findings indicate that AST can serve as an indicator of HAdV RI severity.
5. Discussion
In many children, acute respiratory infections are caused by viruses (15). The incidence of severe pneumonia induced by HAdV is higher compared to common pathogens. HAdV infection primarily affects the respiratory system, often resulting in complications and mortality, which can lead to irreversible and potentially life-threatening sequelae, imposing significant economic burdens on families and society. The initial clinical symptoms of the disease lack specificity, and in the absence of specific drugs or vaccines for treatment, the disease can progress rapidly. Therefore, early identification and diagnosis of severe cases, along with prompt treatment, are crucial in reducing mortality and disability rates.
In this study, we analyzed the clinical data of 665 children with RIs caused by HAdV to investigate the relationship between AST and disease severity. We found that lower respiratory infections accounted for 81.2% of cases, with a death rate of 0.2% among patients. These findings are consistent with the investigation conducted by Chau et al. in Hong Kong (16). The results revealed that 89.8% of the 665 children were under 6 years of age, suggesting that children in this age group are more susceptible to infection, which aligns with previous findings (17). This indicates that susceptibility to adenoviral RI decreases as children grow older. The study by Pereira also demonstrated an increased HAdV immunity in children aged > 5 years (18). In our research, age was identified as a significant factor influencing the severity of HAdV RI, particularly in the severe pneumonia cohort.
Our study further revealed that patients with coinfections involving mycoplasma and HAdV RI, as well as those with longer hospital stays, were more likely to develop severe pneumonia, consistent with our findings (19). The rate of mixed mycoplasma infections in our study was 6.3%. It is reasonable to assume that coinfection with mycoplasma can prolong the clearance time of the pathogen and exacerbate the host's immune response, leading to longer-lasting inflammation.
We have determined that the serum AST level can serve as an independent risk factor for assessing the severity of HAdV RI, as demonstrated in both univariate and multivariate logistic regression analyses. We observed that for every 1U/L increase in AST, the risk of developing severe disease increased by 3.2%. Most adenovirus subspecies utilize the coxsackievirus-adenovirus receptor, primarily located on the cardiac cell membrane. AST is a mitochondrial enzyme present in the cytoplasm and mitochondrial matrix of cells. It is expressed in cells of major organ systems such as the heart, liver, and kidneys. When the human body becomes infected with adenovirus, the coxsackievirus-adenovirus receptor synergistically contributes to cardiomyocyte degeneration, necrosis, and lysis, leading to an increase in myocardial enzyme and AST levels (20). Unlike ALT, AST represents non-specific injury, and elevated AST levels may result from damage to other organs (21). These findings support the association between AST levels and the severity of HAdV RI, consistent with the results reported by Lai (5).
The findings of this study reveal that levels of HGB and serum ALB were significantly lower in children with severe disease compared to those with mild disease. Severe HAdV infection leads to the release of viral presence inflammatory factors, initiating a systemic inflammatory response that damages vascular endothelial cells, reduces blood cell counts, and causes serum ALB leakage. In severe cases, this can result in multi-organ failure and circulatory metabolic disorders. These results are consistent with previous studies (22-24). Spaeder and Fackler also observed that children with severe HAdV pneumonia had elevated AST levels and decreased ALB levels, which were indicative of liver damage caused by adenovirus infection (25).
In our study, a high serum level of CK, CK-MB, and LDH was associated with the severity of adenovirus respiratory infection. Wu et al. found similar results in their study, indicating that LDH could be used as a predictor for the severity of adenovirus respiratory infection, which aligns with our findings. LDH is a cytoplasmic cellular enzyme present in virtually all major organ systems. Elevated LDH levels occur when lung tissue is damaged (26). We observed higher platelet counts in cases of severe HAdV infection, which differs from Chen et al.'s study reporting decreased platelet counts in children with HAdV infection. Their study showed a significant reduction in platelet count in the severe group compared to the mild group, and lower platelet counts were correlated with more severe HAdV RI (27). The discrepancy between our findings and theirs may be due to differences in sample size, overall number of patients, and the number of severe cases included in the study, which can affect statistical analyses. In summary, our results suggest that factors such as HGB, PLT, ALB, LDH, CK, and CK-MB levels can serve as indicators reflecting the severity of HAdV infection.
AST plays an essential role in assessing the severity of RI, yet there are no reports describing its diagnostic efficacy in this regard. Liver function tests are routinely used to assess disease severity due to their ease of collection. In our study, we examined the correlation between AST levels and clinical symptoms. We observed a significant difference in AST levels between cases of severe pneumonia and those with mild RI. Furthermore, ROC curve analysis revealed that the largest AUC was at the AST cut-off value of 52.5 U/L, with 60.6% sensitivity and 83.4% specificity. Wu et al. (26) found that a high serum LDH level (AUC = 0.87) and a low lymphocyte count (AUC = 0.70) could predict the severity of adenovirus respiratory infection in children. The cut-off values for serum LDH level and LYMPH count were 507.5 U/L and 0.39 × 109/L, respectively. Our results also support the association of severe adenovirus infection with LDH, which may be related to the weaker immunity of younger children, and another possible mechanism is that adenovirus may trigger a cytokine storm. The levels of AST and LDH in the severe group were significantly higher than those in the mild group, consistent with the findings of Xu et al. (28). Patients with severe pneumonia may experience both diminished pulmonary ventilation and pulmonary ventilation, which can result in hypoxemia and severe microcirculatory disruptions (29). Severe HAdV RI primarily affects the lungs, leading to a systemic inflammatory response syndrome. Both pathogenic microorganisms and numerous inflammatory factors cause varying levels of damage to liver cells and cardiomyocytes, increasing cell membrane permeability and, consequently, serum AST concentrations (30). Additionally, in a report on markers for the early diagnosis of severe adenovirus infection, the area under the ROC curve of WBC, N, and CRP was 0.748, 0.770, and 0.740 (31). Therefore, our study demonstrates that the AUC of AST in ROC analysis to predict the severity of HAdV pneumonia was 0.782, significantly higher than that of other inflammatory indicators.
Currently, there are no specific features distinguishing early HAdV RI from other RIs, making the identification and assessment of potential RI severity in clinical practice challenging. AST serves as an effective indicator for assessing disease severity. Importantly, its utilization does not add to the medical cost and enhances clinical capabilities.
Our study has three limitations. Firstly, the sample size, especially the number of severe cases, was relatively small, and a larger population is required to validate our conclusions. Additionally, the clinical data were collected from a specific hospital setting, limiting the external applicability of our findings. Thirdly, due to the retrospective nature of the study, pre-admission treatments and disease duration were not considered since this data was not readily available. Therefore, a well-designed prospective study is needed for further confirmation.
5.1. Conclusions
In conclusion, our evidence suggests that children with adenoviral RI are typically under 6 years of age. We have established that AST levels can serve as a stand-alone predictor of disease severity in children with HAdV RI. Hence, early monitoring of AST levels would be valuable for clinicians in accurately and promptly assessing the potential progression to severe disease in children with HAdV RI.