1. Background
Premature infants are at an increased risk of death and morbidity due to the immaturity of the entire body system, particularly the lungs (1). Respiratory distress is the most often encountered problem in preterm infants and the most frequently encountered reason for neonatal intensive care unit (NICU) admission (2). Respiratory distress is a symptom of respiratory issues that includes tachypnea, retractions, nasal flare, and grunting with or without substantial cyanosis (2, 3). It can develop into respiratory failure and cause high morbidity and mortality, and the risk is higher in preterm infants (4). Respiratory failure is a condition in which the respiratory system fails in oxygenation or carbon dioxide elimination (5). This condition then causes acidosis, hypercapnia, and hypoxia (6).
Noninvasive respiratory support, such as nasal continuous positive airway pressure (NCPAP), was the first line for neonates with respiratory distress (7, 8). The progression of respiratory distress to respiratory failure in neonates with NCPAP (NCPAP failure) increases the need for mechanical ventilation on the first day of life (6, 9). Diagnosis of the causes of respiratory distress and respiratory failure was determined by clinical, X-ray, and laboratory investigations, such as blood gas analysis (BGA) (10).
Indonesia was one of the top 5 countries in the list of low-income developing countries with the highest preterm birth (India, China, Nigeria, Bangladesh, and Indonesia). Preterm birth in Indonesia in 2015 was 15.5 in 100 births, which made Indonesia ranked 9th out of 11 countries with preterm births above 15% and ranked 5th out of 10 countries with the highest premature births (11). Hasan Sadikin General Hospital in West Java is one of the national referral centers in Indonesia with a high preterm birth rate and high referred cases of preterm and its complications from tertiary health facilities, especially with respiratory distress.
In limited-resource countries, such as Indonesia, many cases are referred to national centers for advanced support caused by limited health facilities. Monitoring of critically ill infants is based mainly on clinical observation because limited electronic monitors are available.
In Indonesia, Downes scores are the accurate and easiest measurement that is used to determine the severity and monitoring of respiratory distress in neonates (12). The latest research in 2022 showed that the Downes score has a higher prediction efficiency in general evaluation than the Silverman-Anderson Score (SAS) (13). Hourly assessment was very useful for evaluating the progress of respiratory distress.
Different interpretations are shown in various sources. Downes score by the United States Agency for International Development (USAID) Indonesia stated that a score of <4 means no respiratory distress, 4 - 7 shows the presence of respiratory distress, while >7 shows the threat of respiratory failure (14). According to PONED (Pelayanan Obstetri Neonatal Emergensi Dasar/Basic emergency obstetric and newborn care), a Downes score of ≤ 3 shows mild respiratory distress, a score of 4 - 5 shows moderate respiratory distress, and a score of ≥ 6 shows severe respiratory distress and the threat of respiratory failure (15). In both classifications above, the absence or presence of cyanosis is determined by the minimum 40% oxygen. A study by Winda I shows that Downes score > 3 significantly prompted CPAP failure (P = 0.001; odds ratio [OR] = 2.11; interval Kepercayaan [IK] = 95%, 1.69 - 7.67) (16).
A study by John BM in 2015 showed that a Downes score 4 had a sensitivity of 59%, a specificity of 77.39%, and positive predictive value (PPV) of 50% in predicting the use of mechanical ventilation with an OR of 4.94 (95% confidence interval [CI]: 2.35 - 10.39) and a Downes score 3 with an oxygen saturation of 89% at the start of the examination related to the respiratory support need in the first 72 hours (17). A study by Downes, Winda I, John and guidelines by USAID, PONED did not show the increment of Downes score as a risk of respiratory failure. In Indonesia, there has still been no study that showed an effect of the increment of Downes score in 24 hours and the risk of NCPAP failure.
2. Objectives
The aim of this study was to measure the association of Downes score at birth, ages 2, 6, 12, and 24 hours, and the risk of NCPAP failure in the first 72 hours using survival analysis. Moreover, this study could estimate the best time for referred neonates with respiratory distress based on the Downes score.
3. Methods
This prospective observational cohort study was performed to evaluate the Downes score at birth, ages 2, 6, 12, and 24 hours, and assess the presence of respiratory failure within 72 hours. This study was conducted within March to May 2019 at Dr. Hasan Sadikin Hospital in Bandung, West Java, Indonesia, and employed a consecutive sampling method. The inclusion criteria were all neonates of 28 - 36 weeks gestation with respiratory distress (retractions, tachypnea, cyanosis, grunting, and/or impaired airflow) and NCPAP as respiratory support. This study identified and excluded neonates with major congenital anomalies, birth weight less than 1000 g, and respiratory failure at birth or required intubation before two hours of age. This study was approved by the Research Ethical Committee of Hasan Sadikin General Hospital, Bandung, West Java. The sample size was calculated using the rule of thumb formula (N=(n×k)/P, N [sample size], n [number of independent variables = 4], k [constant = 10], P [the prevalence of respiratory failure in neonates 28 - 36 weeks = 3 - 35%]). According to this formula, a minimum sample size of 121 neonates is required in this study.
All study subjects (neonates with respiratory distress) were treated according to the applicable management procedures in Dr. Hasan Sadikin Hospital using NCPAP. All neonates less than 32 weeks gestation get early CPAP (even before respiratory distress occurs) with the initial setting of NCPAP 7, FiO2 starting at 30%. In the initial setting of NCPAP 7, the fraction of inspired oxygen (FiO2) started at 30%, and changes in pressure and FiO2 were adjusted for clinical respiratory distress (Downes score). Respiratory distress monitoring was carried out using the Downes score periodically at birth, 2, 6, 12, and 24 hours. The change in the Downes score was recorded in the monitoring sheet; then, periodic monitoring was continued, and the time of occurrence of respiratory failure was documented.
Respiratory failure in this study was based on the following:
(1) Clinical criteria (Downes score as mentioned in Table 1)
(2) Blood gas analysis examination (Umbilical cord blood artery pH < 7.12 at birth)
(3) Treatment with intubation and mechanical ventilation
| Score | 0 | 1 | 2 |
|---|---|---|---|
| Respiratory rate/min | < 60 | 60 - 80 | > 80 |
| Cyanosis | None | At room air | With 40% O2 |
| Retractions | None | Mild | Moderate-severe |
| Grunting | None | Audible with stethoscope | Audible without stethoscope |
| Air entry | Clear | Decreased | Barely audible |
The data were analyzed using SPSS statistical software (version 25.0) with a significance level of P < 0.05 and 95% CI. Descriptive data were shown in percentages. Survival analysis with Kaplan-Meier and Cox regression analysis was used to analyze the association of Downes score in the first 24 hours and the risk of NCPAP failure in the first 72 hours in neonates of 28 - 36 weeks gestation with respiratory distress.
4. Results
The study was conducted in a prospective cohort within March to May 2019 on premature infants aged 28 - 36 weeks of gestation who were born at Dr. Hasan Sadikin Hospital with respiratory distress. A total of 121 infants met the inclusion criteria from 353 premature infants aged 28 - 36 weeks who were born during the study period, and 132 cases experienced respiratory distress (37.4%).
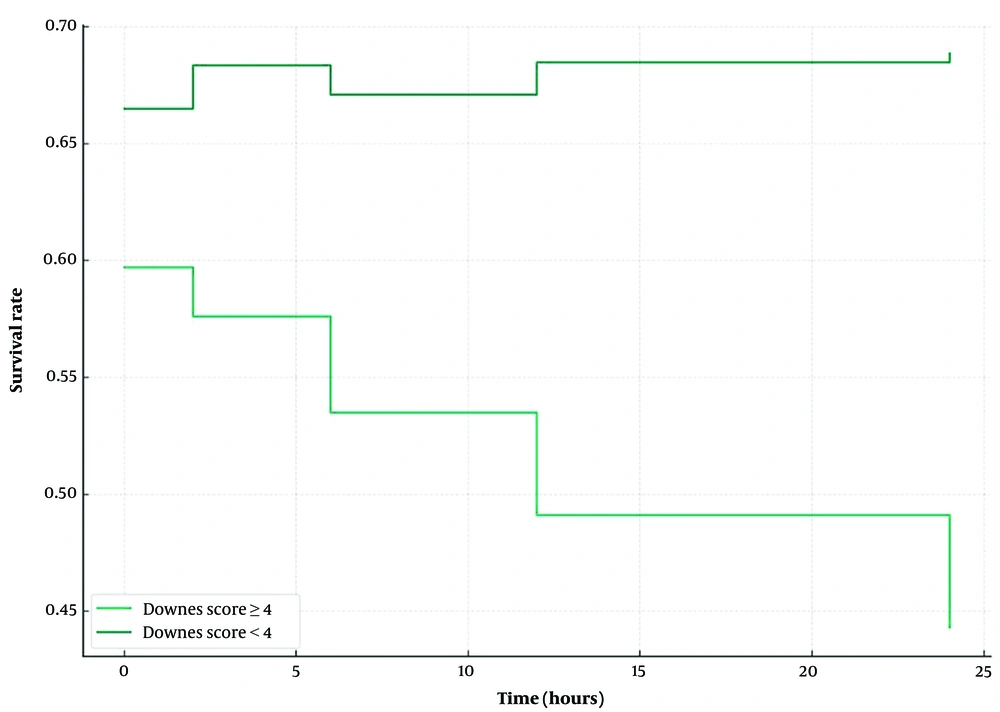
The characteristics of the study subjects are shown in Table 2. Thirty-six neonates (29.7%) in the study experienced respiratory failure with a median gestational age of 30 weeks. A total of 22 neonates (61.1%) with a birth weight of 1000-1499 g experienced respiratory failure, with a mean of 1417.3 g. The most common cause of respiratory failure in this study was respiratory distress syndrome. Table 3 and Figure 1 show the survival characteristics of the research subjects with respiratory failure using the Kaplan-Meier test. Neonates with respiratory distress had a survival rate of 70.2% with a mean of 61.1 hours; in other words, as many as 70.2% of neonates with a gestational age of 28 - 36 weeks with respiratory failure did not experience respiratory failure; however, the rest experienced respiratory failure (29.8% neonates) in the first 72 hours of age, with mean time onset of 61.1 hours.
| Characteristics | Respiratory Failure ≤ 72 Hours (n = 36) | Without Respiratory Failure (n = 85) |
|---|---|---|
| Gender | ||
| Male | 19 (52.7) | 38 (44.7) |
| Female | 17 (47.22) | 47 (55.3) |
| Gestational age (w), median (range) | 30 (28 - 36) | 32 (28 - 36) |
| 28 - 32 | 25 (69.4) | 30 (35.3) |
| 32 - 34 | 8 (22.2) | 26 (30.6) |
| 34 - 37 | 3 (8.3) | 29 (34.1) |
| Birth weight (g), mean ± SD | 1417.3 ± 335.8 | 1700.29 ± 381.2 |
| 1000 - 1499 | 22 ± 61.1 | 29 ± 34.11 |
| 1500 - 2499 | 13 ± 36.11 | 54 ± 63.52 |
| > 2500 | 1 ± 2.7 | 2 ± 2.3 |
| Type of delivery | ||
| Cesarean section | 22 (61.1) | 52 (61.1) |
| Spontaneous head | 13 (36.1) | 31 (36.5) |
| Spontaneous bracht | 0 | 2 (2.3) |
| Forceps extraction | 1 (2.7) | 0 |
| Steroid antenatal | ||
| Complete | 17 (47.2) | 11 (12.94) |
| Incomplete | 5 (13.8) | 11 (12.94) |
| None | 14 (38.8) | 63 (74.11) |
| Cause for respiratory distress | ||
| Respiratory distress syndrome | 35 (97.2) | 68 (80.00) |
| Transient tachypnea of the newborn | 1 (2.7) | 16 (18.82) |
| Others | 0 | 1 (2.63) |
| Sample Size | Number of Events | Number of Sensors | Survival Rate in Hours (95% CI) | %Survival | |
|---|---|---|---|---|---|
| Whole sample | 121 | 36 | 85 | 61.1 (57.6; 64.6) | 70.2 |
| Gestational age, w | |||||
| 28 < 32 | 55 | 25 | 30 | 57.5 (49.4; 60.7) | 54.5 |
| 32 < 34 | 34 | 8 | 26 | 63.94 (58.06; 69.81) | 76.5 |
| 34 < 37 | 32 | 3 | 29 | 66.87 (61.35; 72.39) | 90.6 |
| Birth weight, g | |||||
| 1000 - 1499 | 51 | 22 | 29 | 57.47 (51.69; 63.24) | 56.9 |
| 1500 - 2499 | 67 | 13 | 54 | 64.26 (60.09; 68.44) | 80.6 |
| > 2500 | 3 | 1 | 2 | 53.33 (23.46; 83.20) | 66.7 |
| Gender | |||||
| Male | 57 | 19 | 38 | 58.89 (57.2; 64.5) | 66.7 |
| Female | 64 | 17 | 47 | 63.1 (58.9; 67.3) | 73.4 |
| Birth Downes score | |||||
| ≥ 4 | 96 | 31 | 65 | 59.7 (55.7; 63.8) | 67.7 |
| < 4 | 25 | 5 | 20 | 66.48 (60.83; 72.12) | 80.0 |
| 2 hours Downes score | |||||
| ≥ 4 | 81 | 32 | 49 | 57.6 (52.8; 62.3) | 60.5 |
| < 4 | 40 | 4 | 36 | 68.35 (64.79; 71.90) | 90.0 |
| 6 hours Downes score | |||||
| ≥ 4 | 53 | 24 | 29 | 53.5 (47.2; 59.7) | 52.7 |
| < 4 | 68 | 12 | 56 | 67.08 (63.86; 70.31) | 82.4 |
| 12 hours Downes score | |||||
| ≥ 4 | 46 | 25 | 21 | 49.1 (42.1; 56.1) | 45.7 |
| < 4 | 75 | 11 | 64 | 68.48 (66.02; 70.93) | 85.3 |
| 24 hours Downes score | |||||
| ≥ 4 | 38 | 25 | 13 | 44.3 (36.7; 51.8) | 34.2 |
| < 4 | 83 | 11 | 72 | 68.84 (66.57; 71.11) | 86.7 |
Abbreviations: CI, confidence interval
a Event = incidence of respiratory failure; Sensor = not experiencing respiratory
Table 4 shows that the risk of CPAP failure in 72 hours was increased with higher Downes score at 2 hours (hazard ratio [HR] = 1.86 [95% CI: 1.3 - 2.6], P < 0.001), 6 hours (HR = 1.67 [95% CI: 1.2 - 2.2], P < 0.001), 12 hours (HR = 1.95 [95% CI: 1.4 - 2.7], P < 0.001), 24 hours (HR = 2.27 [95% CI: 1.6 - 3.1], P < 0.001).
| Predictor | B | Crude HR (95% CI) | P-Value | B | Adjusted HR (95% IK) | P-Value |
|---|---|---|---|---|---|---|
| Birth Downes score | 0.27 | 1.31 (1.01; 1.70) | 0.025 | 0.16 | 1.17 (0.89; 1.54) | 0.25 |
| Gestational age | −0.23 | 0.79 | 0.06 | |||
| Birth weight | −0.001 | 0.999 | 0.25 | |||
| 2 hours Downes score | 0.77 | 2.18 (1.61; 2.93) | < 0.001 | 0.62 | 1.86 (1.34; 2.59) | < 0.001 |
| Gestational age | −0.14 | 0.86 | 0.25 | |||
| Birth weight | −0.001 | 0.999 | 0.221 | |||
| 6 hours Downes score | 0.52 | 1.76 (1.28; 2.19) | < 0.001 | 0.49 | 1.67 (1.20; 2.21) | 0.001 |
| Gestational age | −0.23 | 0.79 | 0.05 | |||
| Birth weight | −0.001 | 0.999 | 0.28 | |||
| 12 hours Downes score | 0.82 | 2.3 (1.71; 3.02) | < 0.001 | 0.67 | 1.95 (1.43; 2.66) | < 0.001 |
| Gestational age | −0.16 | 0.85 | 0.22 | |||
| Birth weight | −0.001 | 0.999 | 0.48 | |||
| 24 hours Downes score | 0.84 | 2.32 (1.74; 3.31) | < 0.001 | 0.82 | 2.27 (1.65; 3.13) | < 0.001 |
| Gestational age | −0.48 | 0.61 | 0.002 | |||
| Birth weight | -0.001 | 1.001 | 0.49 |
Abbreviations: HR, hazard ratio; CI, confident interval; B, regression coefficient; IK, interval Kepercayaan.
It was necessary to compare two groups or more (categorical variables) to obtain the average survival with the Kaplan-Meier test, and then the Downes Scores ≥4 and <4 were grouped. Table 5 shows that after controlling for confounding factors of gestational age and birth weight, there is a relationship between Downes score ≥ 4 and the risk of respiratory failure in neonates at 28 - 36 weeks of gestation who experienced respiratory distress and used NCPAP. Downes score ≥4 at 2 hours (3.26 times, P = 0.030), 6 hours (2.44 times, P = 0.014), 12 hours (3.8 times, P < 0.001), and 24 hours (6.93 times, P < 0.001) of age had a high risk of CPAP failure in 72 hours.
| Predictor | B | Crude HR (95% CI) | P-Value | B | Adjusted HR (95% CI) | P-Value |
|---|---|---|---|---|---|---|
| Birth Downes score ≥ 4 | 0.58 | 1.79 (0.69; 4.60) | 0.23 | 0.19 | 1.21 (0.45; 3.19) | 0.702 |
| Gestational age | −0.25 | 0.77 | 0.035 | |||
| Birth weight | −0.001 | 0.999 | 0.26 | |||
| 2 hours Downes score ≥ 4 | 1.55 | 4.71 (1.66; 13.34) | 0.003 | 1.18 | 3.26 (1.12; 9.45) | 0.030 |
| Gestational age | −0.22 | 0.80 | 0.05 | |||
| Birth weight | −0.001 | 0.999 | 0.369 | |||
| 6 hours Downes score ≥ 4 | 1.17 | 3.24 (1.61; 6.48) | 0.001 | 0.89 | 2.44 (1.19; 4.99) | 0.014 |
| Gestational age | −0.20 | 0.82 | 0.095 | |||
| Birth weight | −0.001 | 0.999 | 0.22 | |||
| 12 hours Downes score ≥ 4 | 1.66 | 5.27 (2.58; 10.75) | < 0.001 | 1.33 | 3.80 (1.82; 7.95) | < 0.001 |
| Gestational age | −0.21 | 0.81 | 0.078 | |||
| Birth weight | −0.001 | 0.999 | 0.43 | |||
| 24 hours Downes score ≥ 4 | 2.09 | 8.51 (3.96; 16.62) | < 0.001 | 1.94 | 6.93 (3.36; 14.26) | < 0.001 |
| Gestational age | −0.280 | 0.75 | 0.02 | |||
| Birth weight | −0.001 | 0.999 | 0.43 |
Abbreviations: HR, hazard ratio; CI, confident interval; B, regression coefficient.
5. Discussion
The results of the present study from 121 preterm infants aged 28 - 36 weeks who experienced respiratory distress using NCPAP showed that 36 neonates had respiratory failure (29.7%) with an estimated time of respiratory failure of 60.5 hours. A study by Maria reported that among 57 preterm neonates less than 36 weeks of gestation with respiratory distress receiving NCPAP, 26.3% experienced NCPAP failure in the first 72 hours (18). Another cohort study showed that among 150 preterm neonates with less than 37 weeks of gestation with respiratory distress receiving NCPAP, 37.8% experienced NCPAP failure in the first 72 hours (16). Another study also demonstrated that among 174 preterm neonates of less than 34 weeks of gestation with respiratory distress receiving NCPAP, 37.4% experienced NCPAP failure in the first 72 hours (19). In the present study, the frequency of CPAP failure at 28 - 32 and 33 - 34 weeks of gestation was 64% and 42%, respectively.
Gestational age of 28 - 32 weeks experienced the most respiratory distress and respiratory failure, with a survival rate of 54.5%. This finding is in line with study findings by Gutvirtz et al., which showed that gestational age under 32 weeks increased mortality and morbidity but did not report the age at which death occurred (20).
The most common causes of respiratory distress observed in this study were respiratory distress syndrome (85%) and transient tachypnea in newborns (14%). Neonatal respiratory distress syndrome (RDS) occurs from a deficiency of surfactant due to either inadequate surfactant production or surfactant inactivation in the context of immature lungs. Prematurity affects both these factors, thereby directly contributing to RDS. Respiratory distress syndrome causes hyaline membrane formation in the lungs, making the lungs stiff and poor for gaseous exchange. This disease, in the first 3 days of life, increases the work of breathing and hypoxia, and if not addressed, it causes respiratory failure and/or death (2, 21).
The incidence of RDS is inversely related to the gestational age. The mainstay in the management of RDS involves early continuous positive airway pressure (CPAP), surfactant replacement, and mechanical ventilation if needed. Continuous positive airway pressure provides a distending pressure, which results in better lung volumes and improvement of ventilation‐perfusion mismatch. It might have other benefits, including stretching of the Hering-Breuer reflex, which might improve respiratory drive and result in more regular breathing (22, 23).
Transient tachypnea of the newborn (TTN) was originally described as the clinical manifestation of delayed clearance of fetal lung fluid. Transient tachypnea of the newborn is characterized by a respiratory rate greater than 60 breaths per minute (tachypnea) and signs of respiratory distress (grunting, flaring of nostrils, and intercostal retraction). The clinical features typically appear immediately after birth or within the first two hours of life in term and late preterm newborns. The incidence of TTN can reach up to 13% in late preterm and among term infants delivered by elective cesarean section. Common risk factors for TTN include delivery before 39 weeks of gestational age, precipitous delivery, fetal distress, maternal sedation, and gestational diabetes. The management of TTN is supportive, and standard care with supplemental oxygen might be sufficient. However, non‐invasive respiratory support might be administered to reduce respiratory distress during TTN (24). Continuous positive airway pressure as one of the management techniques for TTN might improve functional residual capacity and facilitate fluid reabsorption. It also guarantees an early and adequate alveolar opening. This might, in turn, improve the work of breathing and gas exchange (25).
According to the results of Cox regression analysis, it was determined that at the age of 2, 6, 12, and 24 hours, a Downes score higher by one point had an increased risk of respiratory failure within 72 hours. This condition is further increased when a one-point higher Downes score is observed at a greater age in hours.
Researchers took the Downes score of 4 according to the guidelines used by PONED and USAID in Indonesia as the cut-off for the definition of moderate respiratory distress that requires breathing assistance. Based on the criteria used by USAID Indonesia, scores of 4 - 7 were categorized as having respiratory distress, and based on PONED, scores of 4 - 5 were categorized as moderate respiratory distress (14, 15). According to the criteria used, the threat of respiratory failure was considered a Downes score > 7, and in previous studies, a Downes score of 4 was used to determine the indication for starting respiratory support NCPAP. A study by Dagar in 2015 showed that a Downes score of 4 had a sensitivity of 59%, a specificity of 77.39%, and a PPV of 50% in predicting the use of mechanical ventilation with an OR of 4.94 (95% CI: 2.35 - 10.39) and a Downes score 3 with an oxygen saturation of 89% at the start of the examination related to the respiratory support need in the first 72 hours (17).
Monitoring Downes score in 24 hours in this study showed that a Downes score of 4 in neonates with 28 - 36 weeks of gestation who experienced respiratory distress with NCPAP more and more quickly experienced respiratory failure. Survival analysis with the Kaplan-Meier test showed that neonates 28 - 36 weeks of gestation with Downes scores start from 4 (>4), having a lower survival rate. Therefore, neonates with a Downes score >4 were found to experience more respiratory failure. Respiratory failure occurs more rapidly when a Downes score of 4 is observed at a greater age in hours.
Using Cox regression analysis showed the relationship of Downes score 4 with the risk of respiratory failure in the first 72 hours in neonates of 28 - 36 weeks gestation with respiratory distress and using NCPAP. In this case, it can be said that at the age of 2, 6, 12, and 24 hours under monitoring, a Downes score of ≥ 4 will increase the risk of respiratory failure 3.26 times (P = 0.030), 2.44 times (P = 0.014), 3.8 times (P < 0.001), and 6.93 times (P < 1.001), respectively, which is statistically significant. Cox regression analysis showed that higher Downes scores, increased Downes scores, and Downes scores ≥ 4 at birth were not statistically significant (P = 0.702) associated with the incidence of respiratory failure. This might be due to the neonatal adaptation process and the variation in lung compliance in the first 2 hours. Pulmonary compliance at the beginning of birth is low; then, with the onset of breathing and lung fluid clearance, it will increase lung compliance in the first 6 hours (26).
This study showed an association between an increase in Downes score with the time of occurrence and the risk of respiratory failure in neonates of 28 - 36 weeks who experienced respiratory distress and used NCPAP. The limitation of this study is that not all confounding factors can be included in this survival analysis.