1. Introduction
Sodium leak channel, non-selective (NALCN) is an ion channel accountable for the background Na+ conductance in neurons (1). Human NALCN is situated on chromosome 13 at location 13q32.3 and is encoded by 44 exons (2). NALCN, along with UNC80, UNC79, and FAM155A, forms the NALCN channelosome (3). This channelosome is involved in neuronal excitability. Under the direct control of hormones and neurotransmitters, the NALCN channelosome participates in the adjustment of the resting membrane potential (4). The activity of NALCN is essential in the balance of circadian rhythm, respiration, locomotion, and pain sensitivity (5). Dysfunctions of the NALCN channelosome lead to a wide range of diseases (3). Heterozygous NALCN variants cause congenital contractures of the limbs and face, muscular hypotonia, and global developmental delay (CLIFAHDD, OMIM 616266). On the other hand, biallelic variants are associated with IHPRF1, OMIM 615419 (6). Herein, we presented 2 IHPRF patients. One was a 1 - year-old girl referred due to constipation and poor weight gain. The other patient was a 2 - year-old girl presenting with hypotonia, constipation, and poor weight gain. In both patients, homozygote NALCN variants were detected. Interestingly, in both patients, a novel variant of c.1434 + 1G>A in NACLN was identified, which, to the best of our knowledge, has not been reported as a pathogenic variant. This work was written according to the case report (CARE) guidelines (7).
2. Case Presentation
2.1. Case 1-Medical History
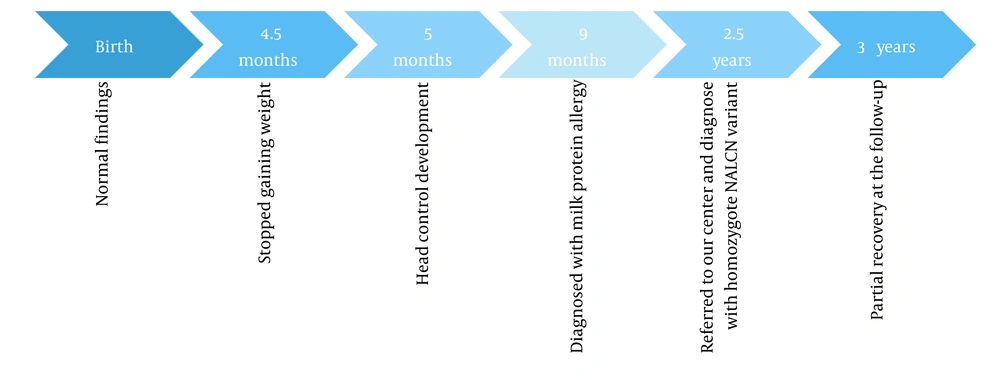
A 1 - year-old girl was referred to our center due to constipation and poor weight gain. Her birth weight was 2.5 kg and had grown to 4.5 kg by 4 months of age, but since then, she has stopped to gain weight. She was the third child of non-consanguineous parents. Her 2 older siblings were healthy. Her perinatal history was unremarkable. She also had a developmental delay. At the end of 5 months, she developed head control, but at the time of admission, she was unable to sit without support or say a word; she only produced vowel sounds. At 9 months of age, the patient was diagnosed with cow’s milk protein allergy and underwent an amino acid diet; however, it did not significantly improve her constipation. In the physical examination, her characteristic features (including long eyelashes, hairy appearance, and high-arched palate) were noticeable. In the abdominal examination, a fecal mass was palpated. The sacral region appeared to be normal. At 2.5 years of age, her head circumference was 41.5 cm, her height was 62 cm, and her weight was 4300 g. Previous diagnostic tests (including urine analysis and culture, thyroid function test, liver function test, anti-tissue transglutaminase antibody, barium enema, and sweat test) were normal. Subsequently, whole exome sequencing was performed for the patient.
2.2. Case 2-Medical History
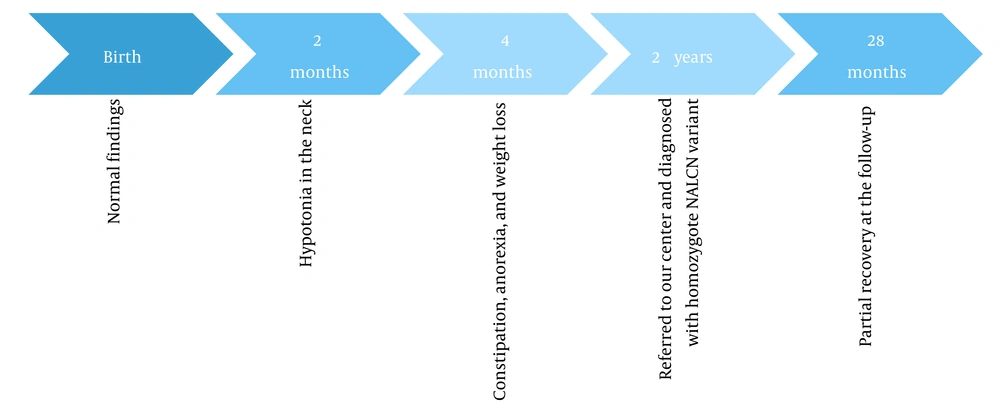
A 2 - year-old girl was referred to our center with a history of cyanosis and severe jaundice during the neonatal period. Her birth weight was 3100 g. She had had hypotonia in her neck muscles since she was 2 months old. At the age of 4 months, she started to develop constipation, anorexia, and subsequently weight loss. She also had a speech delay. In the physical examination, dysmorphic facial features were evident. According to the laboratory findings, sweat NACL was 68 mmol/L, toxoplasma IgG EIA was 1.2 IU/mL, liver enzymes were slightly increased, and metabolic acidosis was reported. On the abdominopelvic ultrasound, a large amount of free gas and bladder debris were reported. Cytogenetic analysis showed a normal karyotype. Table 1 compares the clinical findings of the cases.
| Characteristics | Case 1 | Case 2 |
|---|---|---|
| Age, y | 1 | 2 |
| Gender | Female | Female |
| Ethnicity | Persian | Persian |
| Birth weight, kg | 2.5 | 3.1 |
| Hypotonia | Yes | Yes |
| Psychomotor retardation | Yes | Yes |
| Dysmorphic facial features | Yes | Yes |
| Constipation | Yes | Yes |
| Speech disorder | Yes | Yes |
| Poor weight gain | Yes | Yes |
| NACLN variant | c.1434 + 1G>A | c.1434 + 1G>A |
Comparison of the Clinical Findings of the Cases
2.3. Whole Exome Sequencing
Whole exome sequencing was requested for the patients and was as follows: The patients’ DNA was extracted from their blood samples, with fragments from the coding areas of all known human genes targeted for amplification and sequencing. Next-generation sequencing was performed to sequence close to 100 million reads on an Illumina Sequencer. The bioinformatics analysis of the sequencing findings was conducted using international databases and standard bioinformatics software; in addition, the American College of Medical Genetics (ACMG) guidelines (2015) were applied (8). Variants to the reference were compared against the gnomAD catalog of variants in 123 136 exomes, the Iranome catalog of genomic variations in the Iranian population with 800 healthy individuals, the 1000 Genomes project consortiums publication of 2500 genomes, the NCBI ClinVar database of clinical assertions on variant’s pathogenicity, and several lines of computational evidence on conservation and functional impact. One pathogenic variant in NALCN was detected in the subjects (Table 2). Splice donor variant c.1434 + 1G>A in NACLN (NM_052867.2) has not been reported previously as a pathogenic variant in ClinVar, Iranome, or 1000 Genomes. Its frequency in the general population database is very low (T = 0.000007 [1/139590, GnomAD] and T = 0.00000 [0/10680, ALFA]). This variant mutates a splice donor sequence, potentially resulting in the retention of large segments of intronic DNA by messenger RNA (mRNA) and nonfunctional proteins. The c.1434 + 1G>A variant is a loss-of-function variant in the NACLN gene. Mutation in this gene is associated with IHPRF1.
| Gene and Transcript | Variant | Associated Disease | Zygosity | Inheritance | ACMG Classification | CADD Score |
|---|---|---|---|---|---|---|
| NALCN (NM_052867) | c.1434 + 1G>A | Hypotonia and IHPRF1 | Homozygote | AR | Pathogenic (PM2, PVS1, PP4) | 34 |
Genomic Report
2.4. Treatment and Clinical Outcome
Timelines of events for cases 1 and 2 are shown in Figures 1 and 2, respectively. The patients underwent speech therapy, occupational therapy, and medication. Following the treatment, their motor functions, speech, and constipation improved to some extent.
3. Discussion
NALCN channelopathies are newly discovered disorders, and their clinical aspects in individuals with different variants need further investigation (9). Here, we reported 2 non-related patients with IHPRF1 syndrome, and in both cases, a novel variant of c.1434 + 1G>A in NACLN was detected. The c.1434 + 1G>A variant mutates a splice donor sequence, which can potentially lead to the retention of large segments of intronic DNA by mRNA and nonfunctional proteins. The NACLN gene has a slight rate of benign loss-of-function variants, as demonstrated by a high Z score of 6.07. The c.1434 + 1G>A variant is a loss-of-function variant in the NACLN gene, which is intolerant of loss-of-function variants, as denoted by the existence of pathogenic loss-of-function variant NP_443099.1:p.Y102 and several others. There are 12 downstream pathogenic loss-of-function variants, with the furthest variant being 1105 residues downstream of variant c.1434 + 1G>A. Hence, this variant was interpreted as pathogenic based on the ACMG guidelines (2015) (8).
IHPRF1 is a severe autosomal recessive NALCN channelopathy presenting at birth or in the early weeks of life (10). Similarly, both cases reported here presented clinical symptoms of IHPRF1 in early infancy. The majority of the recessive IHPRF variants are estimated to cause truncated and nonfunctional proteins, leading to a loss-of-function phenotype (11). So far, few cases with homozygous mutations in the NALCN gene have been reported. Most of these cases have been siblings (10, 12, 13). Interestingly in this work, 2 non-related patients showed a similar NALCN variant, which has not been reported so far. IHPRF may present with different phenotypes and even lead to premature death in some patients (11). Variable degrees of hypotonia, psychomotor retardation, and speech disorder ranging from mild to severe have been reported in different cases (10, 12, 14). Male patients may present more severe symptoms than female subjects (15). Some dysmorphic characteristics have been associated with IHPRF 1 syndrome. Angius et al. reported 2 siblings with a wide range of symptoms, including hypotonia, epilepsy, psychomotor retardation, and speech delay. Triangular face, large and constantly opened mouth, high nasal bridge, and bi-temporal narrowing were some features observed in these patients (10). Furthermore, Koroglu et al. reported a prominent forehead, small nose, large and low-set ears, micrognathia, hypoplastic mandible, and strabismus as facial dysmorphism in 2 patients with a recessive mutation in the NALCN gene (13). As shown in Figure 3, dysmorphic facial features were evident in both cases. Constipation has also been proposed as a major presentation of this disorder (15). This symptom was present in both patients in this work. Another presentation was episodes of abnormal respiratory rhythm, as observed by Gal et al. in 2 siblings with a novel homozygous mutation in the NALCN gene (12).
3.1. Conclusions
NALCN dysfunctions cause rare but clinically significant disorders. This work aimed to present 2 IHPRF1 patients with an emphasis on reporting a novel NALCN variant. Due to the potential mortality, further studies are essential to better understand the clinical aspects of these rare disorders.