1. Background
Infant mortality is one of the important indicators of development in different countries, which earnestly try to reduce infant mortality and increase the quality of life of this vulnerable group (1, 2). Currently, neonates are clinically examined by physicians after birth, especially in terms of cardiovascular health, including the presence of patent ductus arteriosus (PDA) (3). The fetal artery duct connects the pulmonary artery and the descending aorta, through which oxygen-free blood returns to the right heart and then is directed to the placenta (4, 5).
Gestational age at labor is inversely related to the spontaneous closure of the ductus arteriosus (DA). Approximately 65% of neonates born between 25 and 28 weeks of gestation and 85% of neonates born in a gestational age of 24 weeks present with PDA in their first week of life (5, 6). In full-term infants, arterial duct naturally contracts after birth, which is stimulated by a rapid increase in arterial oxygen pressure because the DA muscle layer is sensitive to oxygen. It is gradually closed and is eventually transformed into a fibrous non-functional tissue until up to four days after birth; however, anatomically, it may take up to one week after birth for the arterial duct to become closed (7).
Patent ductus arteriosus murmurs are heard more frequently and for longer periods in preterm infants and are typically associated with respiratory distress syndrome (RDS) (7). Various factors such as excessive intravenous fluids, corticosteroids, and prematurity of the infant cause can delay the closure of the arterial canal, leaving it to remain opened longer, disturbing the pulmonary artery and leading to PDA clinical symptoms (7, 8).
Untreated PDA can be associated with side effects such as prolonged mechanical ventilation, bronchopulmonary dysplasia (BPD), pulmonary hemorrhage, necrotizing enterocolitis (NEC), renal failure, intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), cerebral palsy, and even death (8, 9). Due to significant complications of PDA, most infants born to mothers with a gestational age of fewer than 28 weeks will need medical interventions (10). Gold standard for the diagnosis of PDA is cardiac doppler echocardiography, which should be performed by a pediatric cardiologist to accurately detect blood flow through the duct during the cardiac cycle (6, 11).
Echocardiography is the method of choice to diagnose, treat, and follow up neonates with heart diseases. Due to the relatively high cost of the procedure, finding alternative methods is seriously pursued by researchers so that these patients can be diagnosed and monitored in centers with limited facilities (6, 12-14).
Perfusion index (PI) is a non-invasive measure obtained by a pulse oximetry device (15, 16). In recent years, studies have shown that PI can help diagnose some neonatal heart diseases, such as coarctation cases missed by pulse oximetry (17). However, no study has been performed in Iran to evaluate the relationship between PI and PAD.
2. Objectives
Therefore, this study aims to measure PI in infants with PDA and evaluate its agreement with the results of echocardiography.
3. Methods
3.1. Study Design and Inclusion/Exclusion Criteria
This was a case-control study conducted at two hospitals affiliated to the Tehran University of Medical Sciences (TUMS): Neonatal Intensive Care Units of Children’s Medical Center and Imam Khomeini Hospital Complex, Tehran, Iran, from December 2019 to August 2020. Necessary coordination was made with the wards’ staff and their trained nursing teams. Informed consent was obtained from parents after explaining to them the study’s protocol. This study was performed in line with the principles of the Declaration of Helsinki, and ethical approval was granted by the Ethics Committee of Tehran University of Medical Sciences (ethical code: IR.TUMS.IKHC. REC.1398.217).
The statistical population included all neonates with PDA diagnosis who were referred to or hospitalized at the Children's Medical Center and Imam Khomeini Medical Complex. The case group included neonates with significant PDA and without other major cardiac structural disorders. Significant PDA was considered as a shunt size on echocardiography of more than ≥ 1.5, larger than medium absolute PDA size, or the presence of cardiomegaly and pulmonary hyperemia on chest X-ray. Healthy neonates, as the control group, included those who underwent echocardiography because of suspected heart disease but did not reveal any abnormality in echocardiographic examination.
Infants who had a major structural cardiac disorder other than arterial duct and cases whose parents refused to participate in the study were excluded. Repeated echocardiograms were performed by the same pediatric cardiologist who was unaware of the baby’s status of DA closure. If DA remained opened until three months of age, the baby was excluded from the study.
3.2. Data Collection
Considering g-power of 95% and α-error of 5%, the number of subjects in each group was estimated at 19 (total of 38 cases). Neonates in the case group were selected among those who were admitted to the intensive care unit and were diagnosed with PDA by echocardiography during hospitalization. Neonates in the control group were matched with the case group in terms of demographic variables as much as possible. In this study, neonatal demographic characteristics, including the infant’s age, gestational age, weight, and sex, as well as PDA features, were obtained through interviews with parents and reviewing medical records.
O2 saturation (O2Sat) of the patient’s right hand and right foot was measured using a Masimo pulse co-oximeter (Radical-7, USA), and perfusion index was calculated using the following formula:
Perfusion index was determined at a normal temperature (i.e., no hypothermia or hyperthermia), as well as at rest posture. Blood pressure, O2Sat, and PI of the right hand (pre-ductal) and right foot (post-ductal) in the case and control groups were recorded every five minutes for three times. Perfusion index was measured by a trained nurse. Echocardiography was performed by a pediatric cardiologist sufficiently experienced in neonatal heart diseases using a Vivid/GE-S60 echocardiograph (GE Healthcare Co., USA) and a 12/S neonatal probe. The relationship of neonatal PDA with pre/post-ductal O2Sat and PI of the right hand and foot was assessed.
3.3. Statistical Analysis
Data were collected by a researcher-developed questionnaire and analyzed after being transferred into SPSS software (version 21.0 for Windows, IBM SPSS Statistics, Armonk, NY, USA). Descriptive indices such as mean and standard deviation were used to describe quantitative variables. Absolute and relative frequencies were calculated for qualitative variables. Analytical analysis was performed to compare variables between the study groups using Willcoxon and independent t-tests. The significance level was considered P-value < 0.005.
4. Results
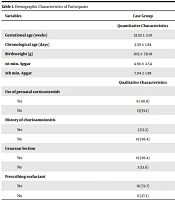
In this study, 44 infants (22 subjects per group) were studied, 13 (59.1%) of whom were boys, and 9 (40.1%) were girls in each of the control and case groups. The demographic characteristics of the participants have been listed in Table 1.
| Variables | Case Group | Control Group |
|---|---|---|
| Quantitative Characteristics | ||
| Gestational age (weeks) | 32.82 ± 3.01 | 35.82 ± 0.95 |
| Chronological age (days) | 2.50 ± 1.84 | 2.18 ± 1.13 |
| Birthweight (g) | 2115 ± 731.81 | 2806.8± 176.8 |
| 1st min. Apgar | 4.90 ± 2.54 | 7.36 ± 0.492 |
| 5th min. Apgar | 7.04 ± 1.88 | 9.04 ± 0.653 |
| Qualitative Characteristics | ||
| Use of prenatal corticosteroids | ||
| Yes | 9 (40.9) | 0 |
| No | 13(59.1) | 22 (100) |
| History of chorioamnionitis | ||
| Yes | 3 (13.5) | 1 (4.5) |
| No | 19 (86.4) | 21 (95.5) |
| Cesarean section | ||
| Yes | 19 (86.4) | 3 (13.6) |
| No | 3 (13.6) | 19 (86.4) |
| Prescribing surfactant | ||
| Yes | 16 (72.7) | 4 (18.2) |
| No | 6 (27.3) | 18 (81.8) |
a Values are expressed as mean ± SD or No. (%).
Alterations in blood pressure, PI, and O2Sat before and after DA closure have been shown in Table 2.
| Variables | Before PDA (Mean ± SD) | After PDA (Mean ± SD) | P Value |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 65.9 ± 12.05 | 72.54 ± 11.38 | 0.021* |
| Diastolic blood pressure (mmHg) | 38.86 ± 8.23 | 46.37 ± 8.61 | 0.028* |
| Right hand’s PI | 0.92 ± 0.36 | 1.2 ± 0.48 | < 0.001*** |
| Lower foot’s PI | 0.93 ± 0.43 | 1.30 ± 0.62 | 0.004** |
| Right hand’s O2Sat | 0.89 ± 0.09 | 0.94 ± 0.44 | 0.002** |
| Lower foot’s O2Sat | 0.92 ± 0.02 | 0.96 ± 0.03 | 0.005** |
There was a significant relationship between systolic and diastolic blood pressure in the case group before and after DA closure. Systolic and diastolic blood pressure in infants before DA closure was low but significantly increased after PDA treatment. Also, PI and O2Sat of the right hand and right foot increased significantly after DA closure. Differences in PI and O2Sat between the right hand and right foot were corrected after DA closure.
Blood pressure, PI, and O2Sat were compared between the case and control groups (Table 3).
| Variables | Case Group (Mean ± SD) | Control Group (Mean ± SD) | P Value |
|---|---|---|---|
| Systolic blood pressure a (mmHg) | 65.9 ± 12.05 | 62.27 ± 4.09 | 0.18 |
| Diastolic blood pressure a (mmHg) | 38.86 ± 8.23 | 41.31 ± 3.12 | 0.44 |
| Right hand’s PI | 0.92 ± 0.36 | 174 ± 0.36 | < 0.001*** |
| Right foot’s PI | 0.93 ± 0.43 | 1.63 ± 0.39 | < 0.001*** |
| Right hand’s O2 Sat | 0.89 ± 0.09 | 0.95 ± 0.00 | 0.006** |
| Right foot’s O2 Sat | 0.92 ± 002 | 0.97 ± 0.00 | 0.04* |
a Blood pressure on cases before PDA closure
Pre-ductal PI, post-ductal PI, and O Sat were significantly lower in the case group than in the control group before DA closure. Based on Pearson correlation, gestational age showed a significant correlation with pre-ductal (r = 0.41, P = 0.005) and post-ductal (r = 0.42, P = 0.004) PI.
5. Discussion
In recent years, researchers have used PI to follow the recovery process of neonates with heart diseases such as PDA (17-20). This study also aimed at finding a simple and accessible method to follow up neonatal PDA by measuring PI as an alternative to echocardiography. The results of this study showed that there was a significant relationship between neonatal systolic and diastolic blood pressure before and after DA closure. Neonatal systolic and diastolic blood pressure were low before DA closure but increased significantly after treatment, which was in line with the results of a study by Bin-Nun et al., who reported elevation of systolic and diastolic blood pressure after DA closure (21).
This study also showed that the mean of pre-closure PI was lower in the case group than in the control group. The difference in mean PI before and after DA closure was significant, which was consistent with the study of Gomez-Pomar et al., who showed that mean pre-PI and ΔPI (the difference between pre-and post-ductal PI) were lower in infants with PDA compared to infants without PDA at the time of echocardiography (22).
According to the results of this study, pre-ductal and post-ductal PI were significantly lower in the case group than in the control group before DA closure. The perfusion index of the right hand and right foot increased significantly after DA closure, and the PI difference between the right hand and right foot was corrected after DA closure. Also, PI increased when tissue oxygenation was improved. This was consistent with the results of some studies reporting that the mean pre-ductal PI and changes in ∆PI in neonates with PDA were significantly lower in neonates with PDA than in their peers without PDA. Pre-ductal and post-ductal PI increased significantly after arterial duct closure, and PI changes on the first and third days of birth were good predictors of PDA in these patients (22, 23). However, according to the results of Vidal et al., PI was noted to be affected by the stability of the duct and the blood flow through it (24).
Our results showed that pre-ductal and post-ductal O2Sat were significantly lower in the case group than in the control group before DA closure. Also, the O2Sat of the right hand and right foot increased significantly after DA closure, correcting the difference in O2Sat between the right hand and right foot. It was also observed that DA closure in 17 neonates led to the elevation of the right hand’s O2Sat. Similar studies also have shown that DA closure increases the right hand’s O2Sat in infants (17, 25). Another study reported that pre-and post-ductal O2Sat were significantly lower in PDA patients compared to healthy neonates; however, O2Sat was not assessed in the two groups after DA closure (25).
In our study, there was no significant difference in systolic and diastolic blood pressure between neonates with or without PDA before treatment, but pre/post-ductal PI in neonates with PDA (before treatment) was significantly different from neonates without PDA. The results of this study showed that there was a significant positive correlation between pre/post-ductal PI and gestational age, which may be explained by the fact that the ducts are open in premature infants. The results of a study by Hu et al. also showed that pre-ductal PI increased as gestational age advanced (26).
This study had some limitations, such as the small number of PDA neonates who had no other significant structural cardiac disorders and the reluctance of some parents to participate in the study. According to our findings, PI in infants with PDA is reduced, which can be corrected by treatment. Therefore, PI can be used as an indicator for evaluating response to treatment and following up patients after treatment where repeated echocardiography is not available.