1. Introduction
Gitelman syndrome (GS) is an autosomal recessive hereditary salt-loss tubular disease characterized by slow growth, hypokalemia, hypomagnesemia, and alkalosis (1). Gitelman syndrome incidence is 1 in 40,000 (2). It is mainly caused by the inactivation mutation of the SLC12A3 gene on chromosome 16 long arm (16q13), whereas some patients are caused by mutations in the CLCNKB gene, with genetic heterogeneity (3). Here, we report a female case of GS caused by the novel compound heterozygous mutations of SLC12A3 accompanied by growth hormone (GH) deficiency (GHD) and subclinical hypothyroidism, which would provide insight into the understanding of the disease.
2. Case Presentation
An 8-year-old girl was referred to our pediatric endocrinology clinic for short stature evaluation. Her height was 113 cm (-2.94SD), and her weight was 16.2kg. The bone age was 6.1 years old. The height of the father and mother was 172cm and 155cm, respectively. She had normal vital signs and a breast Tanner I.
Blood potassium was 3.37 mmol/L (normal range 3.5 - 5.3 mmol/L). The magnesium was 0.46 mmol/L (0.7 - 1.05 mmol/L). The other electrolytes were normal. The urine routine was normal. Liver and kidney function was normal, and bicarbonate was 27.50 mmol/L. The peripheral blood chromosome karyotype was 46, XX. Except for the increase of thyroid stimulating hormone (11.13 mIU/L), all other indicators of thyroid function were normal. The peak GH in the GH stimulation test (clonidine + arginine) was 5.81 ng/mL. The insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein 3 (IGFBP-3) were 97.5 ng/mL and 3.79 μg/mL, respectively. The pituitary MRI was normal. An electrocardiogram was not available. Other blood indicators were normal.
The child began treatment with GH (0.12 U/kg/d, subcutaneous injection, daily) and levothyroxine tablets (12.5 ug/d, oral, daily) on April 4, 2018 (height 113.5 cm).
After 3 months of GH treatment, the height increased to 116.6 cm, but the blood Potassium (3.01 mmol/L) and Magnesium (0.50 mmol/L) decreased. The thyroid stimulating hormone (5.30 mIU/L) increased, but it was better. With blood potassium further decreased, blood gas analysis pH 7.538, HCO3- 20.7mmol/L, alkali residual -4.3 mmol/L, suggesting alkalosis, GS was highly suspected. Urine Potassium was 4.25 mmol/kg/24 h urine (1.03 ± 0.7 mmol/kg/24 h urine).
Then, GH therapy was stopped. The patient began taking potassium and magnesium tablets orally. Blood electrolytes and thyroid function were monitored. The blood potassium fluctuated between 2.79 mmol/L and 4.11 mmol/L (3.5 ~ 5.3 mmol/L). The blood magnesium fluctuated between 0.55 mmol/L and 0.73 mmol/L (0.7 ~ 1.05 mmol/L), and the thyroid function remained normal.
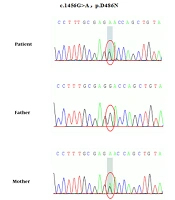
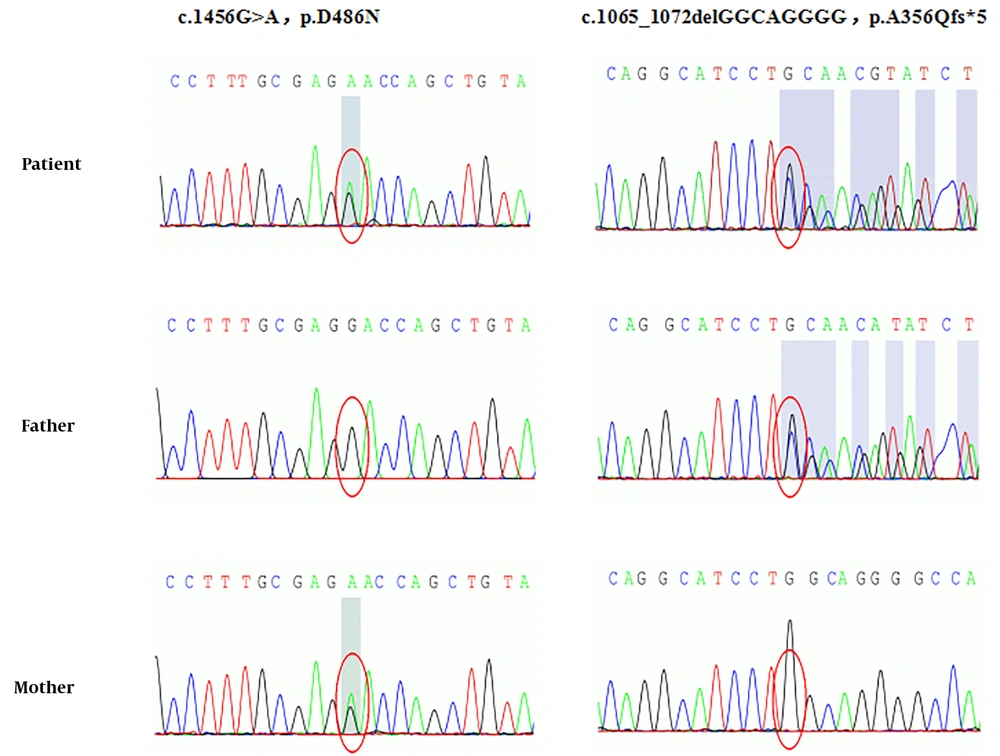
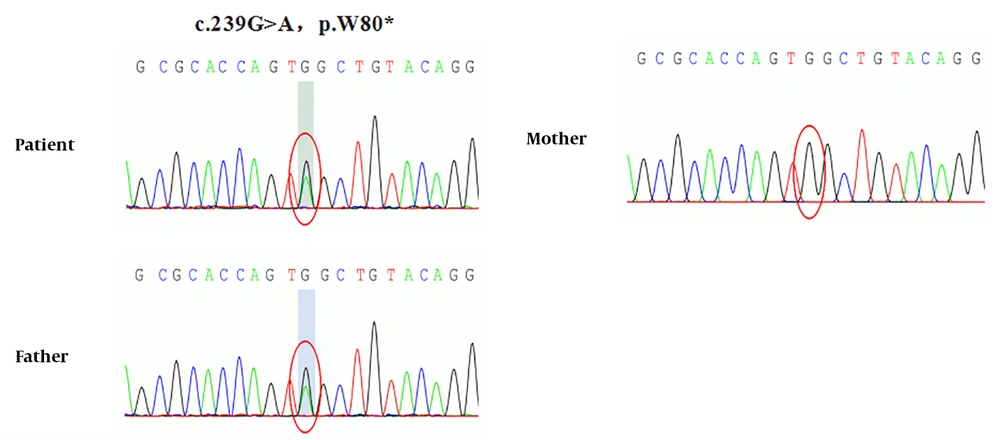
Whole exome and Sanger sequencing revealed novel compound heterozygous mutations in SLC12A3 gene exon regions (Figure 1). One mutation is chr16:56914054. C. 1456G>A (guanine>adenine, missense mutation), which results in amino acid change p.d486n (aspartic acid > asparagine), has been reported as a pathogenic mutation. The patient’s mother was a healthy carrier of this mutation. Another new mutation is chr16:56906668-56906675.c.1065_1072delGGCAGGGG (deletion mutation), which resulted in the early termination of protein translation. This mutation was inherited from the father, who was a healthy carrier. The gene test also showed a heterozygous mutation in the CLCNKB gene (Figure 2). The new mutation is chr1:16373039 c.239G>A (guanine>adenine), resulting in a change in amino acid p.W80* (tryptophan>termination). The patient’s mother was also a healthy carrier of this new mutation.
3. Discussion
GS is common in adolescents and adults and rare in early childhood (4). Before the treatment of GH, the low serum potassium was neglected, and the electrolyte was not rechecked until 3 months after GH, which decreased serum potassium. Therefore, a thorough examination and careful evaluation should be done before treating GH, including blood electrolytes. The blood electrolytes should be monitored monthly for the first three months after treatment of GH. Once the blood potassium level decreases, further diagnosis and treatment should be considered in conjunction with clinical practice to avoid misdiagnosis and mistreatment.
Gene detection is the gold standard for diagnosing GS, and the SLC12A3 gene is the main pathogenic gene. More than 400 different SLC12A3 gene mutations have been reported internationally. Common mutations in different regions are different. For example, IVS9+1G>T splicing mutations are common in Europeans (5), while T60M and D486N mutations are common in Chinese patients (6). Although GS is an autosomal recessive inheritance disease, only 18% of patients have homozygous mutations, 30% have single heterozygous mutations, more than 45% have compound heterozygous mutations, and 7% have three or more mutations (7). In this case, the known pathogenic mutation c.1456G>A (p.D486N) of the SLC12A3 gene originated from the mother, and a new deletion mutation, c.1065_1072 delGCAGG (p.A356Qfs*5), is from the father, which leads to the early termination of protein translation and has a greater impact on protein function. We speculate that these two mutations constitute a new compound heterozygous mutation, which leads to growth retardation in this child.
In recent years, the CLCNKB gene (the main pathogenic gene of Bartter syndrome) also accounts for a certain proportion of the pathogenesis of GS. For example, reports of GS (8) caused by the p.A204T mutation of the CLCNKB gene alone and reports of SLC12A3 and CLNKB mutations in some GS (9). A point mutation was found in the exon region of the CLCNKB gene in this case: chr1:16373039 c.239G>A (guanine>adenine), which resulted in the change of amino acid p.W80* (tryptophan>termination), which may have a greater impact on protein function. Because the CLCNKB gene of this child was a single mutation, it did not constitute a pathogenic disease.
There is little literature on GS with GHD or hypothyroidism, and the mechanism is unknown. A 7-year-old boy and a 9-year-old girl with empty sella and GHD in GS speculated a new and complex hereditary tubular-pituitary syndrome (10). A 16-year-old boy's proband and his 10-year-old sister were both GS (with a c.1456G>A mutation) with severe Graves' disease and thyroid dysfunction (11). Their father, father's uncle, and cousin were carriers of the c.1456G>A mutation (11). They also had mild subclinical hypothyroidism or autothyroid antibodies, and it was speculated that the c.1456G>A mutation in the SCL12A3 gene may affect thyroid function (11). However, in this case, the child's mother is also the carrier of the C. 1456G>A mutation, but her thyroid function is normal. So, the relationship between C. 1456G>A on the SCL12A3 gene and thyroid function needs further analysis. Another report (12) showed an adult patient with GS complicated with clinical biochemical manifestations of GS and hypothyroidism. Because of the clinical symptoms of short stature in this case, it is suggested that the causes of short stature in this child may be GS, partial GHD, and thyroid insufficiency. Combined with the above literature, there are few cases of GS combined with partial GHD and subclinical hypothyroidism; the coexistence of three diseases and the related mechanisms need to be further explored.