1. Background
Neonatal hyperbilirubinemia is a common issue in newborns, occurring in 60% of term neonates and 80% of preterm neonates; nevertheless, it is a transient phenomenon. Although most cases of hyperbilirubinemia are harmless, excessive unconjugated bilirubin can result in death (1). For more than 60 years, phototherapy has been employed to treat hyperbilirubinemia in newborns. Phototherapy’s output spectrum and light intensity are impacted by the shape of the device and lamp. The effectiveness of phototherapy is influenced by the effective wavelength range (460 - 490 nm), light intensity, distance, and body surface area exposed to light (2).
Side effects of phototherapy include loose stools, macular erythematous rash, itchy rash with transient porphyrinuria, overheating, dehydration, hypothermia caused by undressing the newborn, bronze baby syndrome (in the presence of direct hyperbilirubinemia), paralytic ileus, and increasing prevalence of patent ductus arteriosus (PDA) in extremely preterm infants. Studies suggest that phototherapy can be linked with asthma, rhinitis, and conjunctivitis (3), in addition to thrombocytopenia (4) and cancer (5). Additionally, phototherapy can potentially alter the function of cell surface receptors, including growth factor receptors and lymphocyte subunits, and harm the deoxyribonucleic acid (DNA) and immune system of infants, along with impacting cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, 6, and IL-8.
Furthermore, it can worsen immunoglobulin G (IgG) clearance and might lead to the development of humoral immune disorders. There are 5 different isotypes of Ig available: IgM, IgD, IgG, IgA, and IgE, each having a unique structure and function. The essential component for systemic immunity is IgG, which has the highest circulation and longest half-life. A wide variety of infections are prevented by IgG (6). Lower IgG levels might have no symptoms or might increase the risk of frequent infections, autoimmune diseases, allergies, malignancies, and infections, such as Haemophilus influenza and pneumococcus, that affect the airways. In patients with recurrent sinopulmonary infections, Picado et al. reported IgG subclass deficiencies in 8 - 57% of cases (7). There are very few studies conducted on the effect of phototherapy on IgG levels; as a result, no decisive conclusion can be made based on them. The absence of IgG measurement was noted in the studies.
2. Objectives
This study investigated the effects of phototherapy on IgG levels in term icteric newborns, as per the aforementioned materials.
3. Methods
3.1. Study Design and Patients
The present study was conducted at the Neonatal Ward of Children’s Medical Center, Tehran University of Medical Sciences, Tehran, Iran, between March 1, 2021, and March 1, 2022. This study was a prospective investigation that aligned with the American Academy of Pediatrics (AAP) table on full-term newborns with indirect hyperbilirubinemia requiring conventional or intensive phototherapy (8). The phototherapy device was equipped with Tosan-manufactured LED lamps, from Iran, that emitted light with a wavelength of 400 - 500 nm. The lamps were discarded if they were used for more than 2000 hours. In conventional and intensive phototherapy, the distance between the newborn and the lamp was 50 and 15 - 20 cm, respectively. During the study, the neonates were put under phototherapy for at least 72 hours and were allowed to come out of it only for breastfeeding. Two blood samples were taken, one before and one at least 3 days after phototherapy.
Inclusion criteria: A gestational age exceeding 38 weeks, age range from day 1 to day 14 after birth, and meeting the AAP criteria (8) for phototherapy indication
Exclusion criteria: Direct hyperbilirubinemia, treatment with intravenous immunoglobulin (IVIG), packed cells and other blood products or/and exchange transfusion, a positive family history of immunodeficiency, congenital TORCH syndrome, congenital anomaly, albumin prescription, infectious and metabolic diseases, neonates whose parents did not consent to participate in the study, and receiving phototherapy for fewer than 3 days
3.2. Laboratory Tests
The analysis of the blood sample focused on IgG levels. Several items were analyzed, including complete blood count (CBC), bilirubin (total and direct), reticulocyte count, blood group of the newborn and mother, peripheral blood smear (PBS), and glucose-6-phosphate dehydrogenase (G6PD). To test for IgG serum level, 1 cc of venous blood was taken from the neonate and centrifuged at 3000 rpm for 10 minutes at room temperature. Additionally, the serum was separated and sent to the biochemistry department for calorimetry testing. The required amount of serum for testing was 10 landa. Anti-human IgG antibodies, when mixed with samples containing IgG, form insoluble complexes. These complexes cause an absorbance change, dependent upon the IgG concentration of the patient sample, that can be quantified by comparison from a calibrator of known IgG concentration. The implemented kit was AUDIT, Iran, and the IgG levels were read by the auto-analyzer Hitachi 917. It should be noted that for the measurement of this antibody, it was unnecessary to draw more blood from the newborn, and the same amount of blood that was delivered to the laboratory to check for bilirubin was enough to perform the level of this antibody.
3.3. Data Collection
Infants’ characteristics, including gestational age, gender, date of birth, delivery method, weight, number of days of intensive or conventional phototherapy, bilirubin level, and mother’s underlying diseases, were collected. Pre-prepared forms were used to record the data.
3.4. Sample Size
Considering the IgG mean comparing before and after phototherapy in participants and with an effect size of 0.05, margin of error of 0.05, and 85% study power, the sample size was estimated to be 40 participants.
3.5. Statistical Analysis
Mean and standard deviation were used to describe quantitative variables that had normal distribution, and median and interquartile range (IQR) were used for quantitative variables that had skewness. Quantitative variables are presented with frequency and percentage. For comparing the two groups, the Wilcoxon rank-sum test was used. The scatter plot was used to present the IgG levels before and after the phototherapy. Linear regression models were used to evaluate the factors affecting IgG drop in phototherapy. The multivariable linear regression model used all the variables from the univariate analysis with a stepwise approach. The significance levels for univariable and multivariable linear regression were 0.02 and 0.05, respectively. SPSS software (version 25) was used for the analysis of the data.
3.6. Ethics
Informed consent was obtained from the parents of the newborns, and no additional costs or blood sampling was imposed for IgG serum levels on the patients. The confidentiality of the data was maintained. At all stages of the research, the researchers were committed to the principles of the Declaration of Helsinki. The Ethics Committee of the Tehran University of Medical Sciences approved the present study (approval ID: IR.TUMS.CHMC.REC.1400.172).
4. Results
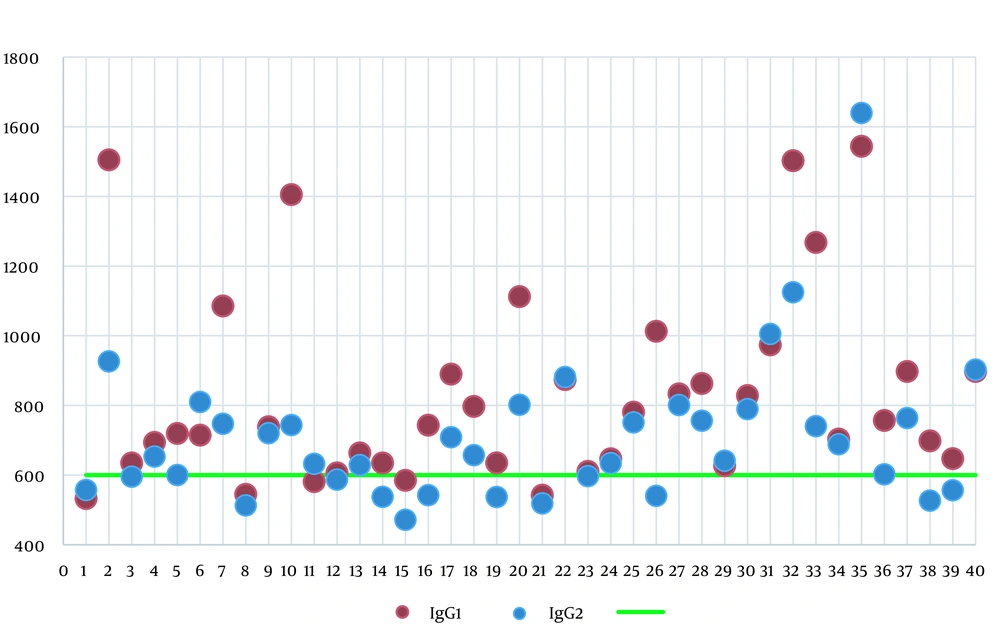
A total of 40 full-term newborns with hyperbilirubinemia were enrolled in the study. In this study, 19 neonates (47.5%) were male. The mean birth weight and age were 3332.13 ± 367.359 g and 6 ± 3 days, respectively. There were three and eight patients with Rh incompatibility and ABO incompatibility, respectively. Four patients were deficient in G6PD. In this study, reticulocyte count was within 0.4 - 7.5%, with an average of 1.9 ± 2.33%. The normal level of reticulocytes based on the neonate’s age on admission showed that 16 patients (40%) had high reticulocytes (9). The IgG level before phototherapy was within the range of 532.5 to 1543.6 mg/dL, with an average level of 833.135 mg/dL. The average IgG level after phototherapy was 720.185 mg/dL, ranging from 471.7 to 1640.3 mg/dL. The normal range of IgG level for healthy neonates based on age is 600-1670 mg/dL (4). Figure 1 shows IgG serum levels before and after phototherapy.
After phototherapy, the paired t-test revealed a significant decrease in the mean levels of IgG serum, hemoglobin, and bilirubin compared to before phototherapy (P = 0.000), as shown in Table 1. Spearman’s correlation coefficient was utilized due to the non-normal distribution of newborns’ weights. The results showed r = 0.11 and P-value = 0.47. No relationship was observed between weight and IgG levels before and after phototherapy. There was a higher decrease in IgG serum level after phototherapy for the group without Rh incompatibility compared to the incompatible group. However, this difference was not statistically significant (P = 0.25) according to the Mann-Whitney U test. The reduction in IgG serum level was higher in the non-ABO blood incompatible group after phototherapy; however, the difference was not significant (P = 0.07) based on the Mann-Whitney U test. The statistical power of the test used was low due to the imbalance between the 2 groups; 8 patients had ABO blood incompatibility; nevertheless, 32 patients had no problems.
| Pair | Paired Samples Test | Paired Differences | t | df | Sig. (Two-Tailed) | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% Confidence Interval of the Difference | ||||||
| Lower | Upper | ||||||
| 1 | IgG1 - IgG2 | 110.4750 ± 202.4804 | 45.7186 | 175.2314 | 3.451 | 39 | 0.001 |
| 2 | Hb1 - Hb2 | 1.2725 ± 1.7118 | 0.7250 | 1.8200 | 4.701 | 39 | 0.000 |
| 3 | Bili1 - Bili2 | 9.6025 ± 2.6252 | 8.7629 | 10.4421 | 23.134 | 39 | 0.000 |
Abbreviations: IgG, immunoglobulin G; Hb, hemoglobin; bili, bilirubin; SD, standard deviation.
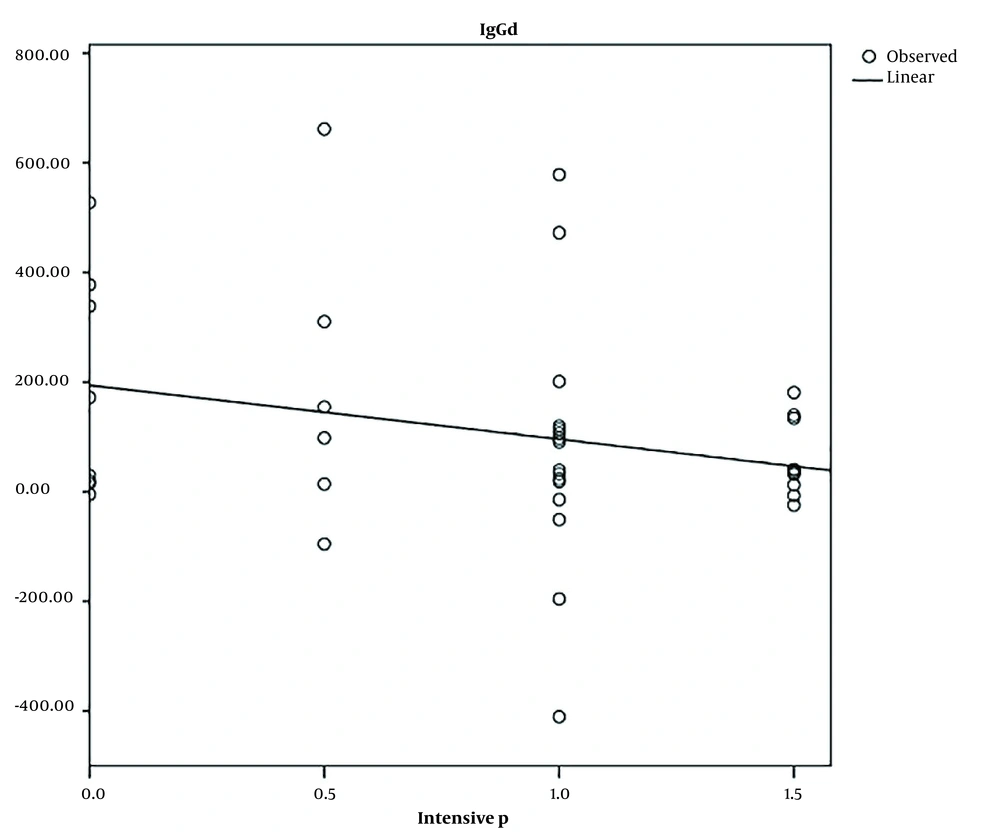
The non-parametric Kruskal-Wallis test revealed a lack of significant difference (P = 0.55) when investigating the correlation between the reduction of IgG serum levels after phototherapy and infant blood groups (Table 2). There was no correlation between age, gender, weight, and blood group with the decrease in IgG level. The regression test revealed a significant correlation between the decrease in IgG and intensive phototherapy (Table 3). The mean IgG was reduced by 0.25 mg/dL for every day of intensive phototherapy (Figure 2). No relationship was observed with conventional phototherapy (Table 3).
| n | Mean ± SD | 95% Confidence Interval for the Mean | Minimum | Maximum | IgG Difference | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| A+ | 18 | 154.8556 ± 221.77546 | 44.5692 | 265.1419 | 95.50 | 661.60 | 65.30 |
| AB+ | 2 | 252.2500 ± 311.48054 | 2546.2916 | 3050.7916 | 32.00 | 472.50 | 252.25 |
| B+ | 8 | 55.4375 ± 143.61573 | 64.6283 | 175.5033 | 96.70 | 338.40 | 13.65 |
| O+ | 11 | 58.9818 ± 184.65689 | 65.0724 | 183.0360 | 377.70 | 410.60 | 97.30 |
| Total | 40 | 112.9500 ± 199.22072 | 49.2361 | 176.6639 | 410.60 | 661.60 | |
Abbreviations: IgG, immunoglobulin G; SD, standard deviation.
| Models | Unstandardized Coefficients | Standardized Coefficients (Beta) | t | Sig. | |
|---|---|---|---|---|---|
| B | Standard Error | ||||
| 1 | |||||
| Constant | 194.074 | 59.466 | 3.264 | 0.002 | |
| Intensive phototherapy | -98.351 | 59.466 | -0.259 | -1.654 | 0.106 |
| 2 | |||||
| Constant | -113.219 | 145.227 | -0.780 | 0.440 | |
| Conventional phototherapy | 73.949 | 46.872 | 0.248 | 1.578 | 0.123 |
5. Discussion
The effect of phototherapy on IgG levels was analyzed in this study. Phototherapy led to a decrease in IgG levels after 3 days. According to the current study, there was a notable correlation between the reduction in IgG and intensive phototherapy. For every day of intensive phototherapy, the average IgG decreased by 0.25 mg/dL. The lack of a relationship in conventional phototherapy might be attributed to the small sample size. There was a smaller decrease in IgG levels in Rh-incompatible neonates than in the compatible groups. The small number of patients with Rh incompatibility (n = 3), compared to those without problems (n = 37), led to an imbalance between the two groups, resulting in the low statistical power of the test used. A study can reveal a relationship by using an equal sample size. The ABO setup groups also show this pattern, and a larger sample size might reveal a clearer pattern in cases of lysis.
Although phototherapy is a safe method for the treatment of neonatal hyperbilirubinemia, it can result in complications, such as macular rash, hyperthermia, loose stools, and dehydration, which can lead to an increase in insensible water loss (3, 10). Studies on the harmful effects of phototherapy on DNA and immune and inflammatory systems are limited (11) and show inconsistent results. Phototherapy has been observed to impact the immune and inflammatory systems of newborns, according to studies. It was reported that the level of white blood cells (WBC) (12), IL-8, IL-1B (13), IL-2r (14), and TNF-α (10, 12) increased after phototherapy. Jahanshahifard et al.’s study (10) demonstrated that phototherapy can enhance the count of WBC and stimulate the release of TNF-α from the peripheral immune system (10, 15).
Studies suggest alterations in lymphocyte expression surface antigens. According to Elfeky et al.’s study, CD3+ and CD19+ lymphocyte percentages decreased in peripheral blood flow cytometry after 72 hours of phototherapy. The patients who had a decrease in CD3+ percentage visited the hospital more often in the 6-month follow-up (16). Kurt et al.’s study showed that phototherapy not only raised the levels of serum TNF-α, IL-1β, and IL-8 but also reduced the percentage of CD3+ (13). Rashedy et al. (17), Eyada et al. (18), and Karabayir et al. (19) showed that phototherapy did not cause any variation in the percentage of lymphocytes in peripheral blood flow cytometry.
Phototherapy can impact both cellular and humoral immunity, causing a decrease in immunity levels through its effect on Igs. Phototherapy decreases Ig levels in newborns, according to Zheng et al. According to the aforementioned study, phototherapy results in an increase in the albumin-to-globin ratio due to globin destruction (6). The long lifespan of IgG is dependent on the neonatal Fc receptor (FcRn), a β2 microglobulin that possesses two IgG binding sites. A possible mechanism for the reduction in IgG levels during phototherapy involves albumin binding to FcRn at a distinct site and increasing IgG degradation (20). Another possible mechanism is photodegradation. Sreedhara et al. demonstrated that protein damage can result from ambient light > 400 nm, typically over a 1 - 7 day period, due to small ultraviolet (UV) emissions (21).
The photodegradation of proteins, including antibodies, was introduced by Wei et al. (22). Antibodies catalyzed the reaction of singlet oxygen with water, resulting in the formation of hydrogen peroxide, known as the antibody-catalyzed water-oxidation pathway (ACWOP). This phenomenon was demonstrated by Wentworth et al.’s (23) group using different antibodies, which produced peroxide after light exposure. The authors suggest that antibodies employ water as an electron source, enabling the combination of singlet oxygen and water to produce H2O3 as the primary intermediate in a succession of reactions that ultimately culminate in the formation of H2O2. Additionally, the authors suggest that the conversion of singlet oxygen to hydrogen peroxide occurred as a result of the conserved tryptophan (Trp) in the antibodies’ buried regions, not the surface-exposed areas (23-25).
The phosphorescence behavior of Trp residues is interesting due to their high flexibility and solvent accessibility (26). Tryptophan possesses solvated strong absorbance in the UV region (260 - 290 nm), making it a target of many photo-oxidation studies (27). Tryptophan is suggested as an endogenous photosensitizer increasing the oxygen-dependent photo-oxidation of tyrosine (28). When Trp is studied through photoexcitation, it leads to the creation of solvated electrons and Trp cation radicals. Solvated electrons reacting with molecular oxygen can result in the formation of superoxide anions. Tryptophan 53 is responsible for the increased efficiency of light energy transfer in monoclonal antibody 1 (mAb-1), and this finding might have a connection to ACWOP as a substrate generator. The pathway leads to site-specific Trp oxidation in mAb-1, which triggers different oxidative degradation mechanisms of other amino acids, including methionines, through photocatalysis (21).
However, the present study was limited by its small sample size and the imbalance between the 2 groups with and without ABO blood incompatibility. Therefore, the findings of this study should be interpreted with caution, and further research with larger sample sizes is needed to confirm these results.
5.1. Study Limitations
The study’s limitations include a lack of long-term patient follow-up and investigation into growth patterns, infection rates, and hospitalization needs for reduced IgG serum levels after phototherapy. Decreasing IgG levels might be due to the reduction of maternal IgG by the age of the newborn, not phototherapy. Only a control group which do not receive phototherapy should answer this question. Therefore, another limitation of this study is the absence of a control group.
5.2. Conclusions
Phototherapy can increase the blood level of bilirubin; however, it might also reduce the body’s immunity. According to the results of the study, intensive phototherapy caused a reduction in IgG levels. Since there was no significant decrease in IgG levels in neonates who received conventional phototherapy, it can be concluded that this treatment is safe in terms of IgG levels.