1. Background
Celiac disease (CD) is a chronic inflammatory enteropathy triggered by gluten (proteins in wheat, rye, and barley) in genetically predisposed individuals who carry the HLA-DQ2/HLA-DQ8 genotype (1). Common symptoms of this disease include pain and discomfort in the digestive tract, constipation or chronic diarrhea, failure of growth and development (in children), anemia, and bruising (2). In addition to systemic effects, CD may impact the development of the dental system and oral mucosa. Oral and dental manifestations of CD include enamel defects (ED), growth delay, recurrent aphthous stomatitis, oral lichen planus, atrophic glossitis, cheilitis, alterations in saliva composition, and reduced saliva volume. The most frequent oral manifestations of this disease are ED and aphthous ulcers (3).
Most studies indicate that ED is significantly more prevalent in the celiac group than in healthy individuals (4-6). However, findings on the frequency of dental caries in CD patients vary compared to healthy individuals. Some studies report no difference in caries prevalence between CD patients and healthy individuals (7, 8), while Costacurta et al. (9) observed a high rate of caries prevalence in CD patients. Conversely, Avşar and Kalaycı (4) found a higher caries rate in healthy individuals than in CD patients.
Studies comparing the quality of life of children with celiac disease to that of healthy children have yielded varied results. In Biagetti et al.'s study, there was no significant difference in quality of life between children with celiac disease and those in the control group (10). However, Lee and Clarke found that the quality of life of celiac patients who exhibited clinical symptoms of irritable bowel syndrome was adversely affected (11). Research specifically focusing on Oral Health-Related Quality of Life (OHRQoL) in celiac patients is limited. In the study conducted by El-Housseiny et al., the OHRQoL score in children with celiac disease was significantly higher than that in the control group (12).
2. Objectives
Considering the limited number of studies in this area, the aim of this study was to investigate the association between the DMFT index, ED, and Oral Health-Related Quality of Life (OHRQoL) in children with celiac disease.
3. Methods
This cross-sectional case-control study received approval from the Ethics Committee of Zahedan University of Medical Sciences (code: IR.ZAUMS.REC.1398.145). The study was conducted from January to April 2020.
3.1. Study Population
To determine the sample size, Power and Sample Size software was used, along with the calculation formula, which is suitable for studies involving one sample and 2 mean tests.
Drawing on the study by Cantekin et al. (2), the standard deviation of DMFT in the case group (σ1) was 2.6, and in the control group, σ2= 1.7. The average DMFT in the case group μ1= 3.5 and in the control group μ2= 2.8. Assuming an alpha of 0.05, 80% power, and a 10% margin of error, the sample size was estimated at 28 for each group. To ensure robustness, 50 children were selected for each group.
The participants were children aged 12 - 15 years diagnosed with CD who had active files at the Celiac Society of Zahedan. Inclusion criteria included being free from other systemic diseases, congenital anomalies, and physical or mental disabilities. Convenience sampling was used to select cases, while controls were chosen from the patients' friends to match the case group in terms of age, gender, and socioeconomic status. Consequently, cases were asked to bring a friend to the dental clinic on the day of the examination. The case group had to be in the ASA I category, according to the American Society of Anesthesiologists. The significance of the research was explained to the parents of both groups through a phone call, and they were requested to accompany their children on the day of the examination. An informed consent form was signed by the parents before the examination.
3.2. Methods and Measurement Tools
Intraoral examinations were conducted by a senior dental student who had received extensive training. The examiner's training was led by an experienced dental researcher and included theoretical sessions on DMFT and enamel defect assessment. This was followed by practical sessions where the examiner practiced on patients under the supervision of a pediatric dental specialist. The calibration process involved the examiner independently evaluating a group of dental patients and then comparing their assessments with those conducted by the experienced dental researcher. This process was repeated until the examiner's results consistently aligned with the standard. Intra-examiner reliability was assessed using the test-retest method; the examiner repeated the DMFT and enamel defect assessments on 20% of the sample 2 weeks later. The consistency of these assessments was measured using Cohen's kappa statistic, which indicated good intra-examiner reliability (kappa coefficient = 0.9).
Each child was examined clinically under appropriate unit lighting, using a dental disposable mirror and explorer, and without drying. The DMFT index was determined according to the World Health Organization criteria (13), and Aine's classification was used for diagnosing ED (14).
To assess Oral Health-Related Quality of Life (OHRQoL), the standard CHILD-Oral Impacts on Daily Performances (CHILD-OIDP) questionnaire was used. This questionnaire, which has been validated and found reliable in the Farsi language (15), consists of 2 parts. The process of completing the questionnaire was carried out in two stages. In the first stage, both controls and cases provided demographic information and answered the first 20 items of the questionnaire. In the second stage, participants completed the CHILD-OIDP index section during a face-to-face interview with the dental student in the waiting room.
Initially, participants were asked to identify any of the eight daily activities listed in the CHILD-OIDP questionnaire that had been affected by their oral and dental problems in the past three months. These activities included eating, talking, brushing teeth, sleeping, remaining calm, smiling without embarrassment, doing homework, and socializing. Next, they were required to rate the severity of the impact of each problem on a Likert scale (1: Low effect, 2: Moderate effect, 3: Severe effect) and to specify the duration of impairment in daily functioning, using a scale of 1 to 3. The CHILD-OIDP score for each daily function was calculated by multiplying the intensity and duration scores, resulting in a possible range of 0 to 9 for each function. The overall CHILD-OIDP score was then determined by summing the scores of all daily functions (ranging from 0 to 72), dividing this total by 72, and multiplying by 100. A higher CHILD-OIDP score indicates a greater impact on oral health problems and a poorer OHRQoL (15).
In addressing potential confounders, our study took into account several variables that could affect the relationship between oral health and quality of life. These variables encompassed age, gender, socioeconomic status, parental education level, and oral health behaviors, including toothbrushing frequency and frequency of dental visits. By accounting for these variables in our analysis, we aimed to reduce their potential influence on the observed association between Oral Health-Related Quality of Life (COHRQoL) and other variables in the study.
3.3. Data Analysis
The data collected from the study were analyzed using SPSSv.20 statistical software. Descriptive statistics were reported using means, standard deviations, and frequencies.
Frequency comparisons between patient and healthy groups were made using the chi-square test. Given the non-normal distribution of the data pertaining to the DMFT index and quality of life indices, a comparison of these indices between the two groups was conducted using the Mann-Whitney U test. The relationship between quality of life and the DMFT index was explored through the Pearson correlation test and simultaneous regression analysis. Additionally, the comparison of the quality of life index related to oral health in children with ED in both the case and control groups was performed using the Mann-Whitney U test.
In this study, a P-value of less than 0.05 was considered to indicate statistical significance.
4. Results
In this study, 100 children aged 12 to 15 years were examined and divided into 2 groups: Case (50 samples) and control (50 samples). In both groups, the proportion of girls was 60%, and boys 40%.
Table 1 presents the results, showing an average DMFT of 2.58 ± 2.39 in the case group and 2.08 ± 1.61 in the control group. According to the Mann-Whitney U test, this difference was not statistically significant (P = .223). The average Oral Health-Related Quality of Life (COHRQoL) in the case group (24.44 ± 19.91) was significantly higher than in the control group (13.35 ± 13.65) (P = .002).
| Variables | Number | Average | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| DMFT | |||||
| Patient | 50 | 2.58 | 2.391 | . 00 | 11.00 |
| Control | 50 | 2.080 | 1.614 | 00 | 7.00 |
| P value | 223 | ||||
| COHRQoL | |||||
| Patient | 50 | 24.44 | 19.919 | .00 | 75 |
| Control | 50 | 13.35 | 13.658 | .00 | 50 |
| P value | 0.002 | ||||
a P-value: The result of the Mann-Whitney U test.
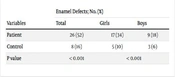
Table 2 indicates that the frequency of ED was significantly higher in the case group at 52%, compared to 16% in the control group (P < .001). Additionally, there was a significant difference in the frequency of ED between boys and girls in the 2 groups (P < .001), with girls with celiac disease exhibiting more ED.
| Variables | Enamel Defects; No. (%) | ||
|---|---|---|---|
| Total | Girls | Boys | |
| Patient | 26 (52) | 17 (34) | 9 (18) |
| Control | 8 (16) | 5 (10) | 3 (6) |
| P value | < 0.001 | < 0.001 | |
aP-value: The result of the chi-square test.
The correlation matrix in Table 3 shows a significant positive relationship between COHRQoL and the DMFT index in both case and control groups (P < 0.001), indicating that an increase in the DMFT index corresponds to an increase in the COHRQoL score.
| COHRQoL | Patient | Control |
|---|---|---|
| Pearson coefficient | 0.653 | 0.671 |
| P value | < 0.001 | < 0.001 |
| No. | 50 | 50 |
Table 4 compares COHRQoL in children with ED in the case and control groups, revealing a significant relationship (P = 0.004). This suggests that ED contributes to an increased COHRQoL score.
| COHRQoL | Number | Average | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| Patient | 26 | 22.17 | 11.252 | 0.00 | 75 |
| Control | 8 | 11.41 | 9.293 | 00 | 50 |
| P value | 0.004 | ||||
aP-value: The result of the Mann-Whitney U test.
Finally, Table 5 details the frequency and percentage of factors causing disruption in the daily functioning of both case and control groups. According to this table, there was no significant difference in the rate of dental caries between the 2 groups.
| What items have they experienced in the last 3 months? | Case Group | Control Group | Chi-Square Value | P Value |
|---|---|---|---|---|
| Toothache | 3.241 | 0.072 | ||
| Yes | 30 (60) | 21 (42) | ||
| No | 20 (40) | 29 (58) | ||
| Tooth sensitive to hot and cold | 1.440 | 0.230 | ||
| Yes | 28 (56) | 22 (44) | ||
| No | 22 (44) | 28 (56) | ||
| Decayed teeth or caries | 1.528 | 0.216 | ||
| Yes | 34 (68) | 28 (56) | ||
| No | 16 (32) | 22 (44) | ||
| Loose deciduous tooth | 0.042 | 0.838 | ||
| Yes | 31 (62) | 30 (60) | ||
| No | 19 (38) | 20 (40) | ||
| Space between teeth due to not erupting a permanent tooth | 0.877 | 0.349 | ||
| Yes | 14 (28) | 10 (20) | ||
| No | 36 (72) | 40 (80) | ||
| Tooth discoloration | 0.932 | 0.334 | ||
| Yes | 13 (26) | 9 (18) | ||
| No | 37 (74) | 41 (82) | ||
| Abnormal place or inappropriate tooth erupting | 0.480 | 0.488 | ||
| Yes | 14 (28) | 11 (22) | ||
| No | 36 (72) | 39 (78) | ||
| Halitosis | 0.794 | 0.373 | ||
| Yes | 16 (32) | 12 (24) | ||
| No | 34 (68) | 38 (76) | ||
| Plaque on the teeth | 1.442 | 0.230 | ||
| Yes | 29 (58) | 23 (46) | ||
| No | 21 (42) | 27 (54) | ||
| Swollen gums | 4.336 | 0.037 | ||
| Yes | 13 (26) | 5 (10) | ||
| No | 37 (74) | 45 (90) |
5. Discussion
In this study, we examined 50 children with CD and 50 healthy individuals, all aged between 12-15 years. The average DMFT was similar in both the case group (2.58 ± 2.39) and the control group (2.08 ± 1.61). This finding aligns with the studies of Perti (16), Shahraki et al. (17), Dane and Gürbüz (18), and Zoumpoulakis et al. (19), where the DMFT rate in both case and control groups was also similar.
However, some studies have reported different findings compared to ours. In the study by Cantekin et al. (2), the average DMFT in the celiac group (3.75 ± 2.62) was significantly higher than that in healthy subjects (1.83 ± 1.78), despite the similarity in DMFT scores between case and control groups in our study. Herwis et al. (20) found that the DMFT rate in CD patients (7 ± 4.4) was significantly lower than in healthy individuals (5.4 ± 6.5). Bıçak et al. (21) observed a significantly lower DMFT level in the celiac patient group. Additionally, the studies by Priovolou et al. (22), Farmakis et al. (23), and Bolguel et al. (24) reported significantly higher caries rates in the control group.
In most studies, the rate of dental caries in cases with CD has been reported as being similar to, or even lower than, that in healthy individuals. A possible explanation for the lower rate of dental caries in celiac patients, particularly in recent years, may be attributed to improvements in patient conditions. Increased awareness and care among families regarding nutritional conditions, oral and dental hygiene, and timely dental visits for these patients could contribute to the reduction in dental caries. Our study was conducted in one of Iran's more deprived provinces, both culturally and economically, which might partly explain why we did not observe a decrease in caries. However, this finding could suggest that the CD itself may not necessarily lead to an increase in caries. It is important to note that caries is a multifactorial disease influenced by many factors. Therefore, we endeavored to select the control group from individuals in close proximity to the person with CD to minimize the confounding effects in the study.
In the current study, ED in CD patients was found with a frequency of 52%, significantly higher than the 16% frequency observed in healthy individuals. Similar findings were reported by Shahraki et al. (17), who demonstrated that ED in the CD group was significantly higher than in healthy individuals. Bıçak et al. (21) found that out of 30 children with CD, 20 had ED in their permanent teeth. Additionally, Acar et al. (25) reported a 37.1% frequency of ED in the celiac group, which was higher than that in healthy individuals. Studies by Priovolou et al. (22) and Farmakis et al. (23) also indicated a high prevalence of ED in celiac patients. The results of the present study are consistent with these findings, suggesting that the presence of ED may be useful for the early diagnosis of CD.
Therefore, identifying ED during a dental examination can serve as an important clinical marker for screening celiac disease. Dentists and dental specialists can play a key role in the early detection and referral of children with undiagnosed celiac disease. Early diagnosis is vital, as it allows for the timely implementation of a gluten-free diet, which is crucial for preventing long-term complications and improving overall health outcomes. Dental screening for ED could help in identifying undiagnosed cases of celiac disease, leading to earlier interventions and improved patient outcomes.
In the current study, the Oral Health-Related Quality of Life (COHRQoL) scores were 24.44 ± 19.91 in the patient group and 13.35 ± 13.65 in the healthy group. During our research, we found only one study that investigated COHRQoL in children with celiac disease (12). However, there are numerous studies on the overall quality of life of children with CD (26-29).
A significant positive relationship was observed between the COHRQoL scores and the DMFT index in this study. This means that an increase in the DMFT index correlates with an increase in the COHRQoL score. In the study by El-Housseiny et al. (12), similar to our findings, the COHRQoL score in children with CD was significantly higher than in the control group.
Treating dental caries can have several benefits for improving the quality of life in affected children. Firstly, restorative treatments can alleviate the pain and discomfort associated with tooth decay. Relief from dental pain can positively impact a child's ability to eat, speak, and perform daily activities, thereby enhancing their overall quality of life. Secondly, treating dental caries helps restore the function and aesthetics of the affected teeth. The impairment caused by tooth loss or structural damage from caries can affect chewing ability, speech, and self-esteem. Appropriate treatment can improve oral function, restore aesthetics, and boost self-confidence, thus positively influencing the child's quality of life.
Furthermore, addressing dental caries early can prevent the disease's progression and the need for more invasive dental procedures. Early intervention and preventive measures, such as fluoride treatments and sealants, can maintain oral health, reduce further decay, and minimize the need for complex treatments. This not only improves the child's oral health but also has a positive impact on their quality of life.
Opinions vary regarding the impact of tooth decay on quality of life. Several studies have identified a clear relationship between dental caries and Oral Health-Related Quality of Life (OHRQoL) (30-34), which aligns with the findings of our study. However, this relationship was not observed in some other studies (35, 36). The discrepancy may be attributed to differences in the statistical populations in terms of age, sex, number, and socioeconomic status. Generally, results derived from epidemiological indicators are heavily influenced by social and cultural characteristics. This is particularly true for health, which is dynamic, multidimensional, and dependent on the environment. Therefore, these differences may arise from variations in the level of disease, culture, access to health facilities, treatment, and accommodations across different societies.
In the current study, a significant relationship was found between COHRQoL and ED. While most studies have not observed a relationship between OHRQoL and ED (37-39), the study by Andrade et al., consistent with our findings, indicated that the presence of ED negatively affects COHRQoL (40).
The findings of this study highlight significant levels of oral and dental problems perceived by children. The most commonly perceived issues in both groups during the last three months were toothache, tooth sensitivity, and decayed teeth. In essence, disruptions in the daily functions of these children were primarily due to problems associated with dental caries.
Castro et al. reported that the problems most clearly perceived by children in Brazil included sensitive teeth (23.2%) and tooth color (42.4%) (41). Similarly, Younessian et al. found that 34.8% of children experienced disruptions in daily functions due to dental caries-related problems. In their study, caries were significantly related to COHRQoL (15), a finding that aligns with the results of our study. Oral and dental diseases are prevalent in children and teenagers, and problems related to dental health or appearance negatively impact their current and future quality of life. These issues can affect daily activities such as playing, sleeping, eating, social participation, and academic performance (27). Arrow and Klobas (42) demonstrated that with 11 months of intervention and treatment of dental caries in children, COHRQoL scores decreased. Jabarifar et al. (27) revealed that a lower DMFT score correlates with better oral and dental health-related quality of life. Therefore, oral and dental problems significantly affect daily life. The study by Younessian et al. (15) also reported that 34.8% of children experienced disruption in daily functions due to dental caries-related problems, and they found a significant relationship between caries and COHRQoL. These studies collectively emphasize the impact of oral and dental health on individuals' quality of life and support the findings of the current study.
According to the results of this study, even though the DMFT (Decayed, Missing, and Filled Teeth) scores were the same in both the case and control groups, the Oral Health-Related Quality of Life (COHRQoL) score was higher in the case group. This suggests a greater impact of oral and dental health on the quality of life in these cases. The variation in COHRQoL scores between the two groups, despite similar DMFT scores, could be attributed to the higher prevalence of ED in the case group. These defects can affect communication, smiling, talking, etc. In this study, ED was found to be significantly more prevalent in girls than boys. Considering girls' heightened sensitivity to appearance, especially in this age group, the impact of ED on their quality of life is understandable.
In a study by McGrath and Bedi (43) conducted in London, which aimed to investigate gender differences in the social impacts of oral and dental health, it was discovered that females, compared to their male counterparts, place greater importance on oral and dental health in terms of quality of life. According to the study, females believe that oral and dental problems can cause toothache, embarrassment, and mood changes. Consequently, they perceive that an improvement in oral health condition results in a greater increase in OHRQoL.
Exploring potential biological mechanisms that could explain the associations between celiac disease, ED, and dental caries can lead to a deeper understanding of the observed relationships. Although the exact mechanisms are not fully understood, several hypotheses have been proposed, including nutritional deficiencies, autoimmune responses, and genetic factors (44).
In this study, the Child OIDP (Oral Impacts on Daily Performances) questionnaire was used. This tool is capable of providing more detailed information related to oral disease indicators than other measures for assessing Oral Health-Related Quality of Life (OHRQoL). As a result, it enhances the evaluation of needs and the planning of oral and dental health services (45).
It is also noteworthy that this study is the only one conducted on OHRQoL in Iranian children with celiac disease. Given the limited research on OHRQoL in children with celiac disease, a more comprehensive and detailed discussion requires further studies with larger sample sizes in this area. Our study employed a cross-sectional design, which limits our ability to establish causality. To determine a cause-and-effect relationship between ED, dental caries, and celiac disease, longitudinal or intervention studies would be necessary.