1. Background
Hypoxic-ischemic brain damage (HIBD), characterized by high morbidity and mortality, is one of the most common neurological diseases that may lead to permanent neuropsychological sequelae (1), including mental retardation, epilepsy, cerebral dysplasia, and cerebral palsy (2). At present, there is no known reliable treatment for HIBD in infants due to the cascade of inflammatory cytokine storms and intricate pathologic mechanisms (3). Investigating an effective therapy that may offer complete functional rehabilitation is necessary.
Brain repeated exposure to mild hypoxia may induce tolerance to hypoxic ischemia and cross-tolerance to other injury factors. A relatively novel concept of mild hypoxia postconditioning was proposed (4). It is based on the same basic rationale as brain exposure, namely the mobilization of endogenous adaptive mechanisms. Mild hypoxia postconditioning was described for the first time in a myocardial ischemia model, and the protective effects on myocardial ischemia have been confirmed by many studies (5-7). Recently, the neuroprotective effects of mild hypoxia postconditioning have been reported by some studies (8). Furthermore, we found that mild hypoxia postconditioning could protect neonatal rats against brain damage and improve neural functional recovery in our preliminary experiment (9). However, how it will work for neonatal brain ischemia and the molecular mechanisms is still less known.
Hypoxia-inducible factor 1 alpha (HIF-1a) is a heterodimeric transcription factor composed of α and β subunits. The α subunit is a functional subunit that regulates HIF-1a activity and is an important transcriptional regulator of metabolic adaptation to changes in the hypoxic environment. The HIF-1a can directly activate hundreds of genes with various functions, including mainly ischemic hypoxia adaptation genes such as vascular endothelial growth factor, erythropoietin, glycolytic enzyme, glucose transferase, etc. Regulating gene expression and participating in many physiological and pathological processes in the body (10), HIF-1a may be neuroprotective against HIBD (11).
2. Objectives
The present study aimed to re-evaluate the effects of mild hypoxia postconditioning on neurobehavior in neonatal rats with HIBD and to determine whether HIF-1a is the key to molecular mechanisms.
3. Methods
3.1. Animals and Hypoxic-Ischemic Brain Damage Model
According to the regional government legislation, the animal experiments were approved by Guangzhou Medical University Animal Ethics Committee. A population of 84 healthy 7-day-old Sprague-Dawley rats from the Laboratory Animal Center of Guangzhou University, Guangdong Province, China (weight = 12 - 20 g), continued nursing with their mothers, were assigned to four groups (n = 21 for per group): The HIBD group, the sham-operated group, the 8% O2 + 92% N2 group, and the 15% O2 + 85% N2 group.
The Rice-Vannucci HIBD model was induced in neonatal Sprague-Dawley rats (12). After successful anesthetization with ether, each rat’s common carotid artery on the left side was exposed, ligated, and severed, and the rats were then placed in an atmospheric pressure chamber filled with 8% O2 and 92% N2 for two hours. After exposure to hypoxia for 60 minutes, the rats returned to a normal oxygen environment and their mothers for feeding. In the sham-operated group, the left common carotid artery was only exposed, not ligated or cut. The animals in this group were not exposed to hypoxia.
3.2. Postconditioning Protocols
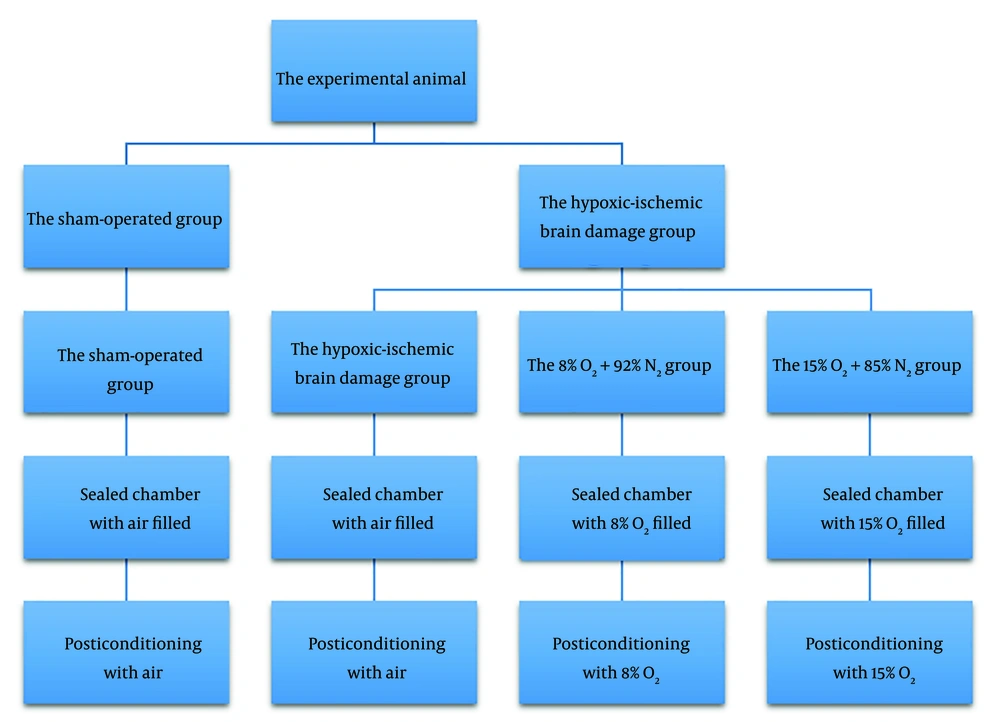
After the animal model was established, the rats were placed in sealed containers, and different concentrations of oxygen were filled into the containers at 9 AM every day for 5 minutes for 5 consecutive days, normoxia for the HIBD and sham-operated groups, and then the stated amount of O2 and N2 for the other two groups. These gas experiments were supplied by the Guangzhou Gas Company, China (Figure 1).
Postconditioning protocols. On the second day of the animal model, the rats were placed in sealed containers filled with oxygen in different concentrations at 9 AM every day for 5 minutes for 5 consecutive days. The chambers were filled with 21% O2 and 79% N2 (air) for the hypoxic-ischemic brain damage group and the sham-operated group, 15% O2 for the 15% O2 + 85% N2 group, and 8% O2 for the 8% O2 + 92% N2 group.
3.3. Immunohistochemistry
On the second day after the postconditioning protocols, sample sections containing the hippocampal area were depicted graphically, re-hydrated, and then cleaned in 0.05 M poly (butylene succinate) (PBS). Each brain tissue section was immersed in antigen unmasking solution and then soaked in 0.3% hydrogen peroxide methanol solution for 20 minutes at room temperature to eliminate endogenous peroxidase. The sections were then incubated with rabbit anti-HIF-1a antibody (1:200, Novus International, NB100-105) overnight in a blocking solution at 4°C. After being washed with PBS, with biotin-conjugated goat anti-rat immunoglobulin G, the sections were incubated at 37°C for 30 minutes, immersed in the catalase complex solution for 30 minutes, in 3-3′ diaminobenzidine for 5 minutes, and then in 0.03% H2O2 for 5 minutes, counterstained with hematoxylin, mounted on a coverslip, and then observed under a microscope. Six slices per rat were analyzed using Image J software (National Institutes of Health, Bethesda, USA).
3.4. Behavioral Tests
The average lifespan of Sprague-Dawley rats is about 36 months. A one-month-old rat is equivalent to a one-year-old child. So, we conducted behavioral tests on about one-month-old rats. Two behavioral tests (platform diving experiment and open field test) were utilized to evaluate the effects of postconditioning exposure on learning and memory performance and anxiety-like behavior. The behavioral tests were carried out on 28-day-old to 30-day-old rats every morning (from 9 AM to 12 PM). (The instrumentation was manufactured by Chengdu Tai-Lian Co., China, and the intelligent video tracking system was provided by Harvard Instruments of Holliston, MA, USA).
3.4.1. Platform Diving Experiment
In order to assess the rats’ learning and memory abilities, platform diving experiments were conducted on 28-day-old and 29-day-old rats. The diving platform equipment with a reflex box was divided into five separate small rooms with black plastic sheets. The placement of copper shutters at 0.5-centimeter intervals was connected to 36-volt current electricity and then fixed on the bottom of the equipment. A non-conductive platform with no electric stimulation was positioned in the back left corner of each separate small room. The rats were acclimatized for the diving platform equipment for 3 minutes. The copper shutters were then charged with a 36-volt current. The frequency of jumping down to the copper shutter or on the non-conductive platform on the first day within 5 minutes was recorded. After 24 hours, this test was repeated on each rat. Differences in memory were investigated by comparing the latency between rats receiving an electric shock and first jumping on the copper shutter. Meanwhile, the mistake frequencies of the rats jumping from the non-conductive platform down to the shutter over 5 minutes were assessed.
3.4.2. Open Field Test
The open field test was applied to examine the anxiety-like behavior of 30-day-old rats. The apparatus comprised a 120 cm × 120 cm × 40 cm white plexiglass box divided into 20 cm × 20 cm blocks. Rats were tested separately for 5 minutes to quantify their walking ability and how far they walked in the peripheral and central squares. The rats were acclimatized to the environment before the test began and placed in the apparatus’s center. Their actions were video recorded by a camera fixed above the instrument. The video camera with a light lamp was fixed above the central squares. The distance moved by each rat in the peripheral squares was calculated as previously described.
3.5. Data Analysis
Data were analyzed using SPSS version 19.0. The data were shown as mean ± standard deviation, and the intergroup comparisons were performed using one-way or two-way analysis of variance (ANOVA). We compared multiple independent samples via the Tukey/Kramer multiple comparisons test. Values less than 5% probability were considered significant.
4. Results
4.1. Mild Hypoxia Postconditioning Increased Hypoxia-Inducible Factor 1 Alpha Expression in the Brain
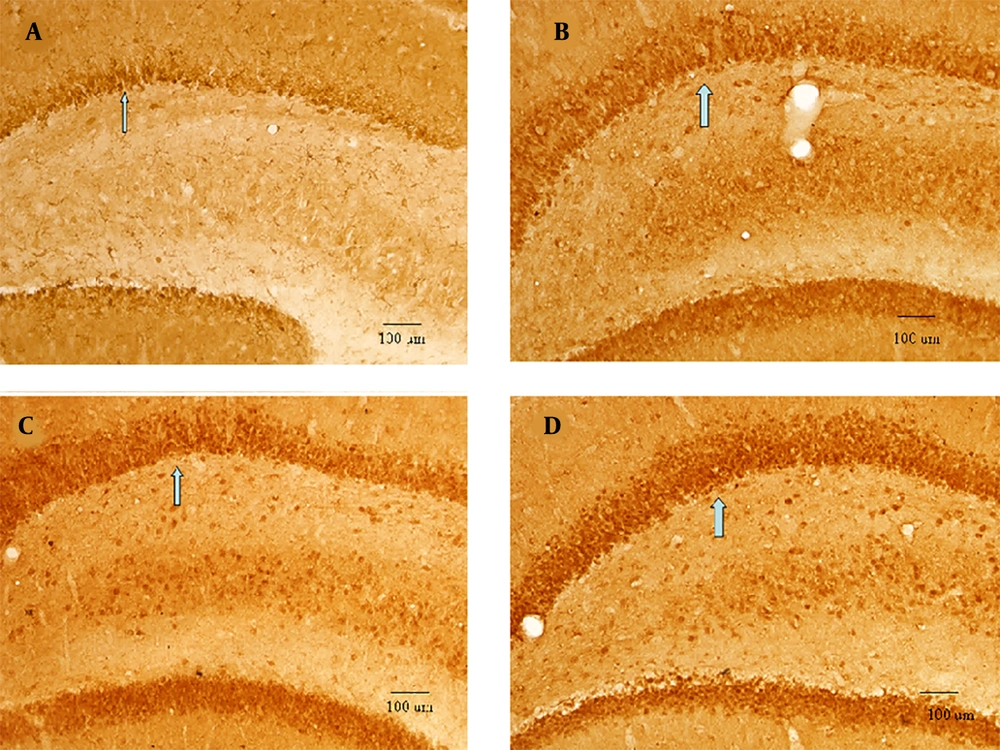
Immunohistochemistry results revealed that HIF-1a expression increased significantly following HIBD (the HIBD group vs. the sham-operated group, P < 0.001). The expression level of HIF-1a further increased following repeated mild hypoxia postconditioning (15% O2 + 85% N2 treatment vs. either 8% O2 + 92% N2 treatment or HIBD, P < 0.05) (Figure 2 and Table 1).
The hypoxia-inducible factor 1 alpha expressions. The hippocampal area of 13-day-old rats (n = 6); the hypoxia-inducible factor 1 alpha immunohistochemistry expression, positively stained cells shown by arrows, EM × 100, SD = 100 μm. A, the sham-operated group: Positive cells were detected to be weak; B, the hypoxic-ischemic brain damage (HIBD) group: Positive cells increased significantly; C, the 8% O2 +92% N2 group: More positive cells than the HIBD group; meanwhile, positive cells peaked in the D, 15% O2 +85% N2 group.
| Protein | Groups | Statistics | ||||
|---|---|---|---|---|---|---|
| Sham-Operated | HIBD | 8% O2 + 92% N2 | 15% O2+85% N2 | F | P | |
| HIF-1a | 41.56 ± 7.19 | 72.50 ± 9.94 | 87.36 ± 12.03 | 131.53 ± 22.87 | 373.98 | < 0.0001 |
The Amount of Hypoxia-Inducible Factor 1 Alpha Expression
4.2. Mild Hypoxia Postconditioning Enhanced Learning and Memory Capacity
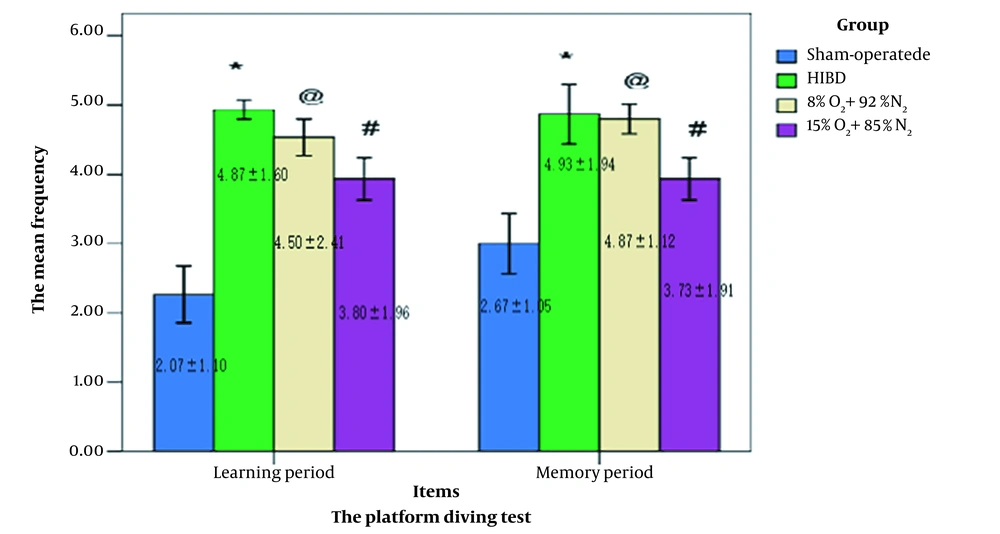
The platform diving experiment was conducted in both 28-day-old and 29-day-old rats to assess their learning and memory capacity. Time spent exploring the target platform did vary between the four groups. At the learning session, the test data showed that the rats in the sham-operated group had lower frequency values than the other groups. In contrast, the rats in the normoxia-treated HIBD group had higher copper shutter frequency values than other rats. There was no significant difference between the HIBD and the 8% O2 + 92% N2 groups (P > 0.05). In contrast to the rats from the 8% O2 + 92% N2 group, the rats from the 15% O2 + 85% N2 group were better able to stay on the platform rather than repeatedly jumping down onto the copper shutters. Moreover, the statistically significant data from the memorizing session was consistent with those from the learning session (Figure 3).
The platform diving experiments were conducted on 28-day-old to 29-day-old rats (n = 15). The repeated mild hypoxia postconditioning improved learning and memory abilities. Two-way analysis of variance for repeated measurements was followed by the Tukey/Kramer test compared with the sham operation group. *: P < 0.001, and the hypoxic-ischemic brain damage (HIBD) group #: P < 0.05, @: P > 0.05.
4.3. Repeated Mild Hypoxia Postconditioning Had Anxiolytic-Like Effects
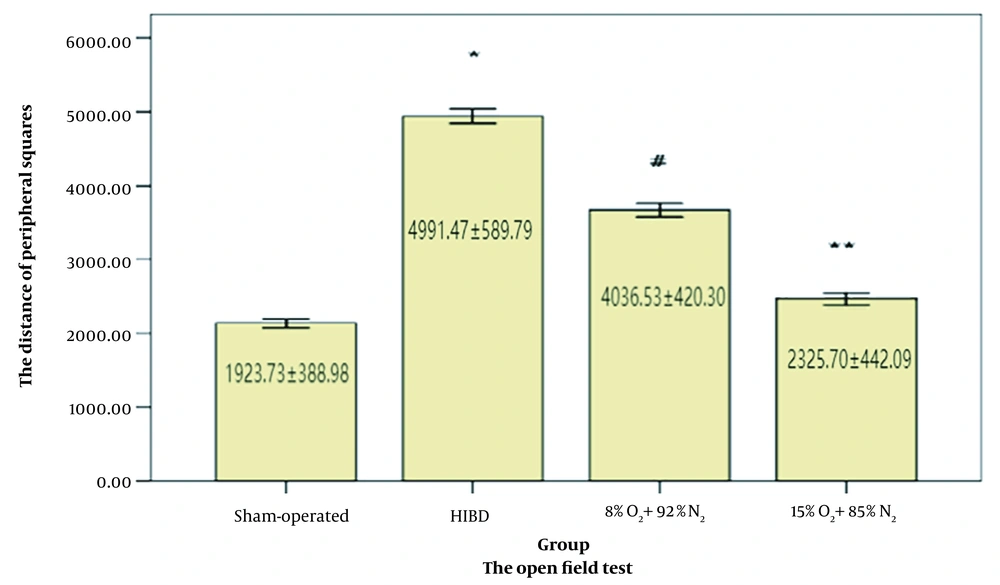
We performed the open field test to evaluate the mild postconditioning effects on the anxiety-like behavior of the rats. The rats moved similarly in the central and peripheral squares. The measured behavioral patterns had the same meaning. The sham-operated group remained quiet in the peripheral squares during the test. However, the rats from the HIBD group demonstrated the most hyperactivity. Although the rats trained with 15% O2 were relatively active, they were significantly quieter than those trained with the 8% O2 (Figure 4).
The open field test was conducted on 30-day-old rats (n = 15). Repeated mild hypoxia exposures tend to shorten the peripheral distance. One-way analysis of variance with repeated measurements was followed by the Tukey/Kramer test. *: P < 0 .001 vs. the sham-operated groups, #: P < 0.05 vs. the hypoxic-ischemic brain damage (HIBD) group, **: P < 0 .001 vs. the HIBD group.
5. Discussion
Based on previous research, the main finding in our study further validates that mild hypoxia postconditioning enhances learning and memory capacity and has an anxiolytic-like effect on neonatal rats with HIBD. It also suggests that HIF-1a may be a critical potential mediator of the effects. To the best of our knowledge, this paper shows for the first time that mild hypoxia postconditioning improves neurobehavioral in neonatal rats with HIBD, and HIF-1a may be a key potential mediator of the observed effects.
Mild hypoxia postconditioning is defined as a fast intermittent series of repeated mild hypoxic-ischemic events after cerebral injury leading to neuroprotection against brain ischemic injury by activating the brain’s endogenous adaptive mechanisms (13). A new supportive option is to enhance postconditioning neuroprotective effects, which should reduce the production of free radicals and also have anti-inflammatory and anti-apoptotic actions (14). Recently, the postconditioning protective effects on brain ischemia have been reported by some studies (15, 16). However, many problems remain to be addressed, such as the neuroprotective mechanisms of postconditioning, the effective window period, effective oxygen concentration, the efficiency procedure, and clinical feasibility. It was found in the current study that mild hypoxia postconditioning would protect neonatal rats against brain damage and improve neural functional rehabilitation under certain conditions.
There are different views regarding the effective oxygen concentration. Teo et al. have reported that 8% O2 hypoxia postconditioning improves the behavioral capacity of neonatal rats following HIBD (17). Serebrovska et al. have also reported that 12% O2 hypoxia training improves cognitive functions in patients with mild cognitive impairment (18). Additionally, Rybnikova et al. have reported that mild hypoxia postconditioning reduces rat brain injury following severe hypoxia (360 torr, 2 hours, three trials spaced 24 hours apart) (19). Nonetheless, we subjected the animals to mild hypoxia postconditioning at oxygen concentrations of 8% and 15% and found that postconditioning with either of them would protect neonatal rats against brain damage and improve neural functional rehabilitation. The neuroprotective effect of 15% O2 was better. Therefore, we confirmed that hypoxia postconditioning might have a wide oxygen concentration range in the neonatal rat brain, as reported by Leconte et al. (20).
As mentioned earlier, studies with different durations of postconditioning yielded different results. Rybnikova et al. have reported that mild hypobaric hypoxia postconditioning (360 torr, 2 hours, three trials spaced at 24 hours) has novel neuroprotective effects (21). Teo et al. have reported that postconditioning (8% O2 for 1 hour/day for 5 days) can reduce inflammatory mediators and cell death after a neonatal HIBD (14). We explored that hypoxia postconditioning would have a 5-minuteefficacy for 5 days. The present findings indicate that repeated mild hypoxia postconditioning (5 minutes, normobaric hypoxia, lasting for 5 days) improves learning and memory and attenuates anxiety-like behavior in neonatal rats. However, the study merely evaluated a brief span of hypoxia postconditioning. Many issues should be resolved, and further experiments are needed.
Some research has been conducted on the neuroprotective molecular mechanisms of mild hypoxia postconditioning. Cao et al. have reported that vascular endothelial growth factor expression links hippocampal activity to neurogenesis, learning, and memory (22). Zhu et al. have reported that hypoxia induces hippocampal neurogenesis and produces antidepressant-like effects in adult rats (23). A few studies have suggested that HIF-1a may play a neuroprotective effect during the repair process of brain damage, and it might be a potential key mediator of the above-mentioned effects (10, 11). The HIF-1a regulates up to 70 downstream genes, such as vascular endothelial growth factor, angiogenesis, erythropoietin, etc. (24). In our study, we measured the levels of HIF-1a and indicated that the sustained expression was achieved following repeated mild hypoxia postconditioning. The increased expression of HIF-1a led to significant neuroprotection against cerebral injury and behavioral consequences in rats subjected to HIBD. This study was fairly preliminary. The elucidation of the mechanisms underlying the endogenous protective efficacy of postconditioning is an important research problem that needs further studies.
5.1. Conclusions
In conclusion, mild hypoxia postconditioning can be neuroprotective against cerebral ischemia injury by mobilizing endogenous adaptive mechanisms. The current research shows that mild hypoxia postconditioning improves learning and memory and decreases anxiety-related behavior in neonatal rats subjected to HIBD. In addition, the results indicate that HIF-1a may be a potential mediator of the neuroprotection effect. These new findings imply that mild hypoxia postconditioning might be clinically feasible for treating HIBD.