1. Background
Device closure of perimembranous ventricular septal defect (pmVSD) is a promising and less invasive method associated with an acceptable success rate (1-8). This method was first performed on congenital muscular ventricular septal defects (VSDs) in 1998 and extended to pmVSD in 2002 (9). However, the incidence of conduction disturbances (CD) remains relatively high during or a few days after the pmVSD closure (9-19). Conduction disturbance can affect the cardiac conduction system and might cause significant hemodynamic effects. Because the exact mechanisms of these abnormalities have not yet been determined, the likelihood of CD after the percutaneous intervention is relatively unpredictable (20). Various hypotheses have been proposed for these irregularities, providing a better understanding of potential factors contributing to CD, some of which are mentioned as follows:
Contact of discs with the myocardium: The percutaneous devices used for closure might come into contact with the adjacent myocardial tissue, leading to electrical disturbances and CDs.
Pressure on the conduction system: The pressure exerted by the device’s waist and its edges on the conduction system during placement could disrupt normal conduction pathways.
Rubbing of the device during myocardial contraction: As the heart contracts and relaxes, the device might rub against the surrounding conduction tissue, causing temporary disturbances.
Progressive device flattening: Over time, the device might undergo changes in shape or become flatter, potentially impacting nearby conduction pathways.
Induction of inflammatory reactions: The procedure itself might trigger inflammatory responses in the surrounding tissues, affecting the conduction system.
Device deformation over time: Long-term changes in the device’s structure might lead to electrical disturbances and alter normal conduction patterns.
Fibrosis of the conductive tissues: The inflammatory response and device-related factors might contribute to fibrosis around the conduction system, further influencing electrical conduction (17).
To further enhance the understanding of the potential mechanisms contributing to CDs after percutaneous pmVSD closure, the present study has included more specific details on these hypotheses. Moreover, a clear research objective at the end of the introduction is provided to better outline the purpose of the study. The present study’s primary aim was to investigate the incidence, risk factors, and outcomes of non-intermittent sustained CDs following successful percutaneous closure of pmVSDs among children and adolescents.
2. Objectives
The primary objective of this study was to determine the incidence, risk factors, and outcomes of non-intermittent sustained CD after successful percutaneous closure of pmVSDs in children and adolescents. By investigating various potential risk factors and exploring the mechanisms underlying CD, this study aimed to provide valuable insights into the management and care of patients undergoing percutaneous closure of pmVSDs. The present study also aimed to establish a clearer understanding of the long-term outcomes associated with this less invasive method.
3. Methods
This prospective cohort study enrolled consecutive patients whose pmVSDs were closed with the antegrade or retrograde percutaneous method in a hospital affiliated with Shiraz University of Medical Sciences, Shiraz, Iran, within April 2016 to April 2021. All the selected patients had successful VSD closure with no complications during the procedures, and the patients were followed for the variables that might affect CD.
This study was approved by the Ethics Committee of Shiraz University of Medical Sciences (ethics code: IR.SUMS.MED.REC.1398.301). All methods were carried out in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from all the participants or from a parent and/or legal guardian if participants were under 16 years old.
This study included children and adolescents less than 18 years and over 5 kg who had successful pmVSD closure and pulmonary vascular resistance less than 6 WU.m2 with at least one of the following criteria:
Pulmonary to systemic blood flow ratio (Qp/Qs) ≥ 1.5, left atrial or ventricular dilatation due to the left to right shunt, recurrent pulmonary infection, failure to thrive, mild aortic valve prolapse, or regurgitation. Patients with residual VSDs and underlying arrhythmias or CDs were excluded from the study.
In this study, sustained CDs were defined and evaluated based on the following criteria to enhance the clarity of the results:
Any CD or arrhythmia that started after releasing the device and lasted more than 30 seconds was considered sustained and included in the study. This duration was chosen to distinguish between transient and more prolonged CDs.
If a second-degree or third-degree atrioventricular block occurred after device insertion but before the device release, the device was not released, and the procedure was stopped. These cases were also excluded from the study to focus solely on the CDs that occurred after successful device closure.
By using the above-mentioned clear and specific criteria, the present study aimed to accurately identify and classify the occurrence of sustained CDs following the percutaneous closure of pmVSDs in children and adolescents.
3.1. Procedures
The pmVSD size, septal aneurysm, and the distance between the VSD and the aortic valve annulus were determined using transthoracic echocardiography and cardiac catheterization. The echocardiography was performed in standard views, including five-chamber, parasternal short, and long-axis views, using transthoracic echocardiography with M-mode, two-dimensional, Doppler, and color-Doppler methods. All the methods were performed according to the guidelines of echocardiography (21, 22).
The diameter of the VSDs was determined from the left ventricular and right ventricular sides. The device diameter was chosen to be 2 mm larger than the VSD size in diastole in both aneurysmal and non-aneurysmal defects.
In this study, Amplatzer occluders type I and II and pmVSD occluder were used for VSD closure. These devices were made by Abbott (Abbott Cardiovascular, MN, USA), Occlutech (Occlutech GmbH, Wildenbruchstr, Germany), Lifetech (Lifetech Scientific Building, Shenzhen, China), and Cardi-O-Fix (Starway Medical Technology Inc., Beijing, China) companies.
Conscious sedation was applied during each procedure. After arterial and venous access, heparin with a dose of 100 u/ kg (maximum 5000 units) and cefazolin with a dose of 50 mg/kg (maximum 2000 mg) were injected. At first, hemodynamic evaluation was performed, including arterial pressure and saturation, left to right shunt, and systemic and pulmonary vascular resistance. Then, left ventricular angiography was mainly performed at about 50° left anterior oblique/30° cranial view.
The type of device was chosen according to the size and location of the defects. The pmVSDs with more than 2 mm aortic rim and the aneurysmal VSDs were closed with symmetric or asymmetric pmVSD Amplatzers or duct occluders type I. The Amplatzers entered the VSDs anterogradely from the inferior vena cava to the right ventricle toward the VSDs.
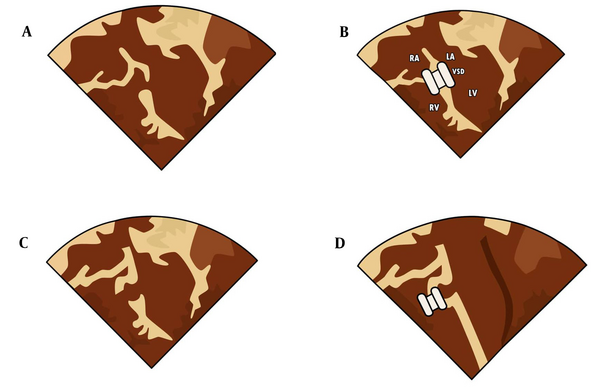
The pmVSDs with aortic rims less than 2 mm were closed in an antegrade manner with eccentric zero-edge pmVSD Amplatzers. Closing the non-aneurysmal VSDs by inserting the left disc of the Amplatzer inside the left ventricle is named the non-aneurysmal VSD closure. The placement of the occluder left disc into the aneurysm is named aneurysmal closure (Figure 1 and 2).
Different forms of ventricular septal defects (VSDs) and various types of devices; (A) and (B) Non-aneurysmal VSD closure with symmetric perimembranous ventricular septal defect (pmVSD) device; (C) and (D) Aneurysmal VSD with three holes to the right ventricle (R.V.) Closed with Amplatzer duct occluder type I; (E) and (F) Aneurysmal VSD closed with Amplatzer duct occluder type II
The smaller diameter of the type I duct occluder was used for statistical analysis. The procedures were performed under transthoracic echocardiography and fluoroscopy guides.
3.2. Post-procedure Care
Continuous heart monitoring was performed for any CDs and arrhythmias during the procedures and one hour after. A standard 12-lead electrocardiogram (ECG) was obtained before starting the procedure, immediately after the end of the procedures, every 6 hours after the closure for 24 hours, 7 days 30 days later, and every 6 months. In addition, any CDs were recorded.
The patients with complete atrioventricular block were admitted to the hospital, and continuous cardiac monitoring was performed and treated with intravenous 0.2 mg/kg/day doses of dexamethasone and 40 mg/kg/day doses of ibuprofen for a week. Other cases of sustained CDs, such as bundle branch block or junctional rhythm, were monitored on an outpatient basis without medication.
The device size ratio (in millimeters) to the body surface area was used to evaluate the effect of the occluder size on the probability of CD. The time of fluoroscopy and cine angiography were recorded to determine any correlation with the occurrence of CD (23).
3.3. Data Analysis
In this study, the data were analyzed with SPSS software version 25.0 (SPSS Inc., Chicago, IL, USA). The mean, standard deviation (SD), frequencies, percentages, and 95% confidence interval (CI) were used for descriptive analysis. Moreover, analytic data were analyzed by the Mann-Whitney U test and Chi-Square test. The logistic regression model was used to evaluate the risk factors for electrocardiography changes. P-values less than 0.05 were considered statistically significant.
4. Results
A total of 260 cases were included in the study. In this study, 135 (52%) and 125 (48%) patients were male and female, respectively. The mean age of the patients was 75.66 ± 68.89 months (range: 9 - 216 months). The median follow-up of patients was 36 months (range: 12 - 71 months), and 31 patients (11.9%) showed sustained CDs. The ECG changes observed after VSD closure were diverse and included several conditions, such as right bundle branch block, left bundle branch block, bi-fascicular block, and junctional rhythm, among others. The demographic, echocardiography, and catheterization data of the cases are shown in Table 1. The type of VSDs and the applied devices are shown in Table 2.
| Characteristics | Mean ± SD |
|---|---|
| Age, mo | 75.66 ± 68.89 |
| Weight, kg | 20.19 ± 14.33 |
| Height, cm | 109.61 ± 26.65 |
| Body surface area, m2 | 0.76 ± 0.34 |
| Echocardiography findings | |
| Diameter of the left ventricular hole opening, mm | 7.15 ± 1.53 |
| Diameter of the right ventricular hole opening, mm | 4.18 ± 1.38 |
| Subaortic rim, mm | 3.19 ± 2.58 |
| Angiographic findings | |
| Diameter of the left ventricular hole opening, mm | 7.22 ±1.48 |
| Diameter of the right ventricular hole opening, mm | 4.14 ± 1.43 |
| Subaortic rim, mm | 3.12 ± 2.78 |
| Occluder size, mm | 8.52 ± 2.21 |
| Corrected occluder size (range mm/m2) | 13.10 ± 6.61 |
| Fluoroscopy time, min | 12.36 ± 7.32 |
| Cineangiography time, sec | 21.84 ± 16.84 |
| Qp/Qs | 2.21 ± 1.31 |
Demographic, Echocardiography, and Catheterization Data of Patients
| Characteristics | No (%) |
|---|---|
| With septal aneurysm | 156 (60) |
| Without septal aneurysm | 104 (40) |
| Left deployment position | 128 (49.30) |
| Right deployment position | 132 (50.70) |
| Occluder type | |
| ADO I | 181 (69.61) |
| ADO II | 24 (9.23) |
| pmVSD occluder | 55 (21.15) |
Characteristics of the Ventricular Septal Defect and Type of Devices
The comparison of demographic data, angiographic data, and devices between the cases with and without CD are shown in Table 3. Table 3 shows that CD is more common in non-aneurysmal defects with the more prolonged procedure and less common using Amplatzer duct occluder type II (ADO II).
| Characteristics | No Conduction Abnormality | Conduction Abnormality | P-Value |
|---|---|---|---|
| Patients | 229 (88.1) | 31 (11.9) | - |
| Gender (male) | 120 (46.1) | 16 (51.6) | 0.592 |
| Age, mo | 78.35 ± 76.31 | 58.57 ± 44.89 | 0.132 |
| Weight, kg | 20.20 ± 14.49 | 16.76 ± 10.93 | 0.149 |
| Height, cm | 108.88 ± 26.64 | 100.64 ± 15.74 | 0.237 |
| Body surface area, m2 | 0.66 ± 0.34 | 0.74 ± 0.21 | 0.085 |
| Echocardiography findings | |||
| Left ventricular hole opening diameter, mm | 7.19 ± 1.98 | 6.87 ± 3.45 | 0.911 |
| Right ventricular hole opening diameter, mm | 4.18 ± 2.88 | 4.16 ± 2.17 | 0.881 |
| Subaortic rim, mm | 4.01 ± 1.23 | 3.51 ± 1.45 | 0.241 |
| Aneurysmal VSDs | 151 (65.93) | 5 (16.12) | 0.043 |
| Angiographic findings | |||
| Left ventricular hole opening diameter, mm | 7.26 ± 2.53 | 7.91 ± 2.69 | 0.738 |
| Right ventricular hole opening diameter, mm | 4.14 ± 2.15 | 5.18 ± 2.52 | 0.787 |
| Subaortic rim, mm | 4.93 ± 1.86 | 3.46 ± 1.14 | 0.113 |
| Occluder size, mm | 8.52 ± 2.94 | 8.50 ± 1.23 | 0.848 |
| Corrected occluder size, mm/m2 | 9.61 ± 3.73 | 12.51 ± 4.11 | 0.097 |
| Fluoroscopy time, min | 11.59 ± 4.12 | 17.87 ± 3.74 | 0.038 |
| Cineangiography time, sec | 21.50 ± 5.21 | 24.25 ± 4.92 | 0.695 |
| Right deployment position | 128 (55.89) | 20 (12.9) | 0.152 |
| Left deployment position | 101 (44.10) | 11 (87.9) | 0.041 |
| Occluder type | |||
| ADO I | 156 (68.12) | 25 (80.64) | 0.156 |
| ADO II | 23 (10.04) | 1 (3.22) | 0.001 |
| pmVSD occluder | 49 (21.39) | 6 (19.35) | 0.251 |
Comparison of Demographic and Procedural Characteristics of Patients with and Without Conduction Abnormalities
Among 31 patients (11.9%) with arrhythmias, right bundle branch block was the most common, which occurred in 16 patients (6%) but resolved in 12 cases. This form of CD was observed to be more common in the aneurysmal type (n = 13) in comparison to the non-aneurysmal type (n = 3) (P = 0.021).
Left bundle branch block was detected in 2 non-aneurysmal cases (1%), which did not resolve during the follow-up. One bi-fascicular block (0.5%), including the right bundle branch block and left anterior hemiblock, was diagnosed in a patient with a non-aneurysmal defect that became permanent. The junctional rhythm was observed in 4 patients (1.5%), including 1 aneurysmal and 3 non-aneurysmal types. It lasted less than 15 minutes, resolved without special treatment, and did not recur during the follow-up.
Three patients (1.2%) with non-aneurysmal defects developed third-degree atrioventricular block, which was treated with corticosteroids. All of them reverted to sinus rhythm in less than 3 days, and none required pacemaker insertion. Five patients (2%) with aneurysmal defects had frequent premature ventricular contractions, which resolved spontaneously after the procedure. Overall, 7 patients had permanent CDs (2.5%), including 4 cases of right bundle branch blocks, 2 cases of left bundle branch blocks, and 1 case of bi-fascicular block in the follow-up.
Longer fluoroscopy time and non-aneurysmal closure were independent risk factors of CD or arrhythmias, and VSD closure with ADO II reduced the risk significantly (Table 4).
| Variables | Logistic Correlation Coefficient | Odds Ratio | P-Value |
|---|---|---|---|
| Fluoroscopy time | 0.11 | 1.11 | 0.032 |
| Left deployment | 2.42 | 11.28 | 0.036 |
| ADO II | -0.93 | 17.23 | 0.001 |
Logistic Regression Analysis of Risk Factors for Conduction Defect After Perimembranous Ventricular Septal Defect Closure
5. Discussion
Conduction disturbance is an important complication of percutaneous pmVSD closure. First-, second-, and third-degree atrioventricular blocks, fascicular blocks, hemiblocks, bradycardia, atrial, junctional, and ventricular CD or arrhythmias were reported (24). The present study showed that CD or arrhythmia occurred in 11.9% of cases with successful pmVSD closure, and most of them reverted to normal conduction; however, 2.5% of all cases developed permanent CDs (Table 4). Conduction disturbances were more common in non-aneurysmal pmVSDs and more prolonged procedures and less common with ADO type II devices.
Some studies showed that most complete atrioventricular heart blocks occur less than 7 days after the procedures, as in the present study; nevertheless, they might occur in the second week or even later and rarely occur one year after the procedure (2, 3). The incidence of the third-degree atrioventricular block is estimated at about 1% to 5%; however, most convert to the normal rhythm (1, 5, 6, 25).
Several potential risk factors of CD or arrhythmia associated with pmVSD closure have been suggested; nevertheless, the exact mechanism is still unclear (24). Individual differences in the conduction system might play a role. Reducing the manipulation and the placement of the device in the aneurysmal defects might be important factors in reducing the risks of CD and arrhythmia (20, 26).
According to a study, mapping the conduction system in patients undergoing transcatheter device closure of pmVSD using a three-dimensional electroanatomic mapping system can help understand the relationship of the conduction system to pmVSD. The course of the conduction system and its relationship with the pmVSD were mapped before and after device closure. The study showed that the course and relation of the conduction system were posteroinferior to the pmVSD in all cases (100%) and away from the defect in 67% (10/15). In patients with baseline right bundle branch block, the right-sided conduction system was in close proximity to the pmVSD. Two patients had a part of a left-sided conduction system in close proximity to pmVSD or device edges. Two patients developed a right bundle branch block following device deployment, which reverted to normal on follow-up. No patient developed high-grade atrioventricular block during the median follow-up of 34 months (range: 24 - 62 months) (27).
Different mechanisms have been discussed for post-device CD. Some transient CDs are supposed to occur due to transient electrical instability of the adjacent cardiac myocytes (3). Others include traumatic pressure of the device on the nearby conduction system, pressure effect of the over-sized device, or induction of inflammatory reactions and fibrosis (20). Some experts have suggested that the appearance of grade two or three blocks during the placement of the delivery system and before fixing the device is potentially a predictive factor of future heart block. In such a situation, surgery is recommended (15, 24). The current study also followed the same policy.
Due to the inflammation near the device, steroidal and non-steroidal anti-inflammatory drugs have been proposed to treat CD to reduce inflammation and localize the edema around the conduction system (15). Although most CDs were not persistent in the present study, other studies have reported more permanent CDs and heart blocks after pmVSD closure (28).
Some studies concluded that lower age, weight, and body surface area are risk factors for CD. In the current study, the mean age, weight, and height were lower in the CD group; however, they were not statistically significant (24). The corrected device size to the body surface area did not play a significant role in the occurrence of CD in the present study, contrary to Zhao et al.’s study (29), which concluded that the corrected device size to the body surface area was an independent risk factor for arrhythmia (9, 17). The distance of pmVSD to the aorta, which was a risk factor for cardiac arrhythmia in Yang et al.’s study, was not statistically significant in the current study (9).
In the present study, it was observed that the fluoroscopy time in the CD group was significantly longer. During the procedure, the mechanical stimulation of the heart with a catheter might play an important role in the occurrence of CD. Premature ventricular contractions and branch blocks, which are common during the procedure, reinforce that mechanical stimulation might be a crucial arrhythmogenic mechanism (9).
To determine the device size, this study measured the narrowest part of the VSD and selected the devices 2 mm larger than it, similar to Ghaderian et al.’s study (25, 26). In a study by Mijangos-Vazquez et al., they used smaller devices, and they did not exceed 1 mm in non-aneurysmal and 2 mm in aneurysmal VSDs that had no significant increase in residual VSD and a lower rate of heart block (30).
In the current study, the right bundle branch block rate was higher in the aneurysmal defects, and the incidence of the left bundle branch block and complete heart block was higher in non-aneurysmal defects. However, due to the small number of patients, statistical analysis was not performed.
Softer devices, such as ADO II, might cause less CD. Some researchers investigated ADO II for pmVSD closure. These Amplatzer devices are softer, more flexible, and have a lower profile, making them easy to install. In addition, these devices might quickly adapt to the VSD shape with less interference with adjacent structures and less CD (28). The findings of the aforementioned study are also valid in the present study, and patients treated with ADO II had less CD than other patients.
The current study showed the lack of statistical significance for certain potential risk factors, such as age, weight, height, corrected device size to the body surface area, and distance of pmVSD to the aorta; nevertheless, some researchers reported them as significant risk factors. Larger studies with longer follow-up periods are recommended to further explore the role of these factors in the occurrence of CDs after pmVSD closure.
Furthermore, the current study has delved into the potential benefits and limitations of using steroidal and non-steroidal anti-inflammatory drugs to treat CDs. These medications have been proposed to reduce inflammation and localize edema around the conduction system, which could have implications for managing CDs after closure. Further investigation into the efficacy and safety of this treatment approach is warranted to offer more comprehensive insights.
5.1. Limitations
Although the present study was prospective, further studies with larger sample sizes and longer follow-up periods, even life-long, are recommended to determine CDs’ incidence and risk factors. Standard 12-lead ECGs were obtained every 6 hours for 24 hours, 7 and 30 days later, and then every 6 months. However, this method is not efficient for detecting intermittent and paroxysmal heart arrhythmia or CD.
5.2. Conclusions
Most CDs and arrhythmias following percutaneous pmVSD closure were benign and self-limiting, and they were mainly temporary and recovered to normal conduction. A delicate performance in this procedure and reduction of the manipulations of the pmVSD hole and the surrounding areas might have a crucial role in preventing CD. Selecting softer devices might also decrease the rate of CD.