1. Background
Swallowing is a coordinated function of the soft palate, pharynx, pharyngeal muscles, epiglottis, and upper esophageal sphincter that transfers food from the oral cavity to the esophagus. This joint mechanism protects the airway from food aspiration (1). Dysphagia is a frequent complication defined as difficulty in any stage of swallowing (i.e., oral, pharyngeal, or esophageal) (2).
A different degree of dysphagia was reported in 25 - 40% of children. This incidence rate is increasing by improvement in the survival rate of preterm infants (3, 4). Prematurity, low birth weight, congenital anomalies, perinatal asphyxia, sepsis, and surgery are the major contributors to swallowing complications (3). It was also reported that infants with a history of mechanical ventilation might show difficulty in coordinating pharyngeal muscles resulting in dysphagia (5). Dysphagia in children is presented through a wide range of signs and symptoms, such as refusal of eating, difficulty in eating foods with different textures, prolonged feeding time, difficulty in chewing or sucking, sneezing or coughing while eating, spitting food out by mouth or nose, low weight gain, and frequent respiratory infection (6). These difficulties might occur during different periods of pre-feeding, feeding, or post-feeding, showing the criteria of abnormalities in the oropharyngeal or pharyngoesophageal phases (7, 8).
Considering the high prevalence of swallowing disorders and their vital importance, early diagnosis, and intervention can significantly influence pediatric health indicators. By timely diagnosis, infants could be referred to the associated specialist and speech therapist to take necessary measures.
2. Objectives
The present study was designed to show the prevalence of swallowing disorders and associated symptoms among high-risk infants with a history of prolonged mechanical ventilation. Furthermore, this study evaluated the potential effects of oral stimulation and speech therapy maneuvers on the frequency of symptoms to show the possible beneficial effects of these interventions. Despite the importance of this issue, to the best of our knowledge, no similar study has been carried out in Iran.
3. Methods
A quasi-experimental study (before/after intervention) was conducted at the Breastfeeding Research Center affiliated with Tehran University of Medical Sciences, Tehran, Iran. The study population was selected from the infants who attended the Growth and Development Follow-up Clinic (Vali-e-Asr Hospital; 2019 - 2021). All the cases with a history of prolonged neonatal mechanical ventilation (longer than 7 days) and delayed initiation of oral feeding entered the study.
The inclusion criteria were an age range of up to 2 years at the start of the study and a ventilation period longer than 7 days. The exclusion criteria were anatomical/structural abnormalities in the pulmonary airways or digestive tract, chronic respiratory disease, neurologic disorders, cardiovascular disease, short-time ventilation period, and the reluctance to participate in the study.
After receiving a signed informed consent, participants’ parents were asked to respond to a provided questionnaire regarding their infants’ possible swallowing disorders. The questionnaire was composed of 9 yes-no items to show the presence or absence of abnormalities in the oropharyngeal phase, including feeding-related choking, coughing, spitting food out by mouth, spitting food out by nose, humid breath, poor weight gain, the need to cut food into small pieces, hospitalization due to food aspiration, or difficulty in swallowing solid foods (8). Any positive answer to each question was considered a swallowing disorder.
According to the parents’ responses, infants with any symptoms were considered candidates for intervention. The parents of infants with dysphagia were referred to the Development Follow-up Clinic to be trained in oral sensorimotor stimulation based on “oral stimulation in speech therapy protocol” to improve infants’ oral sensory-motor skills and reflexes. These interventions were composed of practicing stretches, massages, pressures, and movements to qualify functions of the tongue, lip, gum, cheeks, jaw, soft palate, and muscles. The manipulations of lips, jaw, and oral cavity before feeding with and without nutritive sucking were also considered to enhance neonates’ sucking (9, 10). The parents were also recommended to perform a 10-minute exercise three times daily for their neonates. Parents’ skills related to the implementation of the protocol were also evaluated by a therapist through each follow-up visit. A period of 3 - 6 months after the initiation of the intervention, the questionnaire was filled in for every included infant through a follow-up visit to assess their swallowing statuses.
All infants’ demographic and clinical data, including age, gender, gestational age, weight at birth, hospital discharge, cause of hospitalization, duration of mechanical ventilation, the 1st- and 5th-minute Apgar scores, and brain ultrasound findings, were extracted from medical records. The criteria related to swallowing discomfort before and after intervention were also gathered through history taking and recorded in a checklist. Finally, all the data related to the responses before and after interventions were compared to show the possible effects of the interventions.
3.1. Sample Size
Amantea et al. (11) showed swallowing discomfort in 28% of infants with a history of neonatal mechanical ventilation. Considering a power of 80%, an alpha error of 0.05, missing data of 10%, and presuming that the frequency reaches 0 by implemented intervention, the proposed sample size based on the following formula was calculated at 25.
The data analyses were performed by SPSS software (version 25). The quantitative and qualitative variables were shown in number (percentage) and mean ± standard deviation. McNemar’s test was used to compare the variables before and after the intervention. A chi-square test was used to compare the qualitative variables. The independent sample t-test and Fisher’s exact test were also implemented to indicate the frequency of symptoms, severity of symptoms, and improvement of outcomes based on influential factors, such as gestational age, weight, and Apgar score. The P-values < 0.05 were considered statistically significant.
3.2. Ethical Approval
The present study was extracted from a specialist student’s thesis. Ethical approval for the study was obtained from the Institutional Review Board of Tehran University of Medical Sciences according to the Helsinki Declaration (IR.TUMS.MEDICINE.REC.1398.622). All the patients’ information was also considered confidential, and no extra cost was imposed on the patients.
4. Results
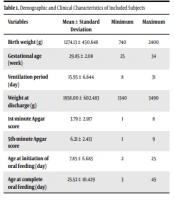
A total of 25 eligible infants (10 females and 15 males) with mean birth weight and gestational age of 1274.13 ± 450.64 g and 29.85 ± 2.08 weeks were randomly entered the study, respectively. The mean mechanical ventilation period was 15.95 ± 6.644 days (min: 8 and max: 31). Table 1 shows the detailed demographic and clinical characteristics of the included subjects.
| Variables | Mean ± Standard Deviation | Minimum | Maximum |
|---|---|---|---|
| Birth weight (g) | 1274.13 ± 450.648 | 740 | 2400 |
| Gestational age (week) | 29.85 ± 2.08 | 25 | 34 |
| Ventilation period (day) | 15.95 ± 6.644 | 8 | 31 |
| Weight at discharge (g) | 1958.00 ± 602.483 | 1340 | 3490 |
| 1st-minute Apgar score | 3.79 ± 2.187 | 1 | 8 |
| 5th-minute Apgar score | 6.21 ± 2.413 | 1 | 9 |
| Age at initiation of oral feeding (day) | 7.85 ± 6.683 | 2 | 25 |
| Age at complete oral feeding (day) | 25.52 ± 10.429 | 3 | 45 |
Demographic and Clinical Characteristics of Included Subjects
Regarding brain-ultrasound findings, 19 patients had different degrees of intraventricular hemorrhage (IVH), including grade 1 with 12 (48%), grade 2 with 6 (24%), and grade 3 with 1 (4%) subjects. Sepsis on admission was also reported in 22 neonates (88%) (9 with early-onset sepsis and 13 with late sepsis). Blood culture in 12 septic cases was positive.
Concerning the frequency of swallowing complications, 24 infants (96%) had different degrees of dysphagia; nevertheless, one case (4%) had no symptoms. The most frequent signs and symptoms were the need to cut food into small pieces (n = 14; 56%), poor weight gain (n = 14; 56%), and food extrusion by mouth (n = 13; 52%), followed by a choking sensation with feeding (n = 9; 36%), humid breath (n = 9; 36%), and coughing with feeding (n = 8; 32%).
The data analyses showed that after a period of 3 - 6 months of interventions, the majority of swallowing complications were relieved among the participants (Table 2). After the intervention period, the number of cases with symptoms of coughing (P = 0.016), spitting food out by mouth (P = 0.0001), choking (P = 0.016), humid breath (P = 0.031), poor weight gain (P = 0.002), and the need to cut food into small pieces (P = 0.004) was significantly lower than the number of cases suffering from such swallowing complications before the intervention. On the other hand, the implementation of oral stimulation could not improve symptoms of difficulty in swallowing solid meals (P = 0.500) or spitting food out by the nose (P = 0.250).
| Variables | Before | After | P-Value |
|---|---|---|---|
| Coughing with feeding | 8 (32) | 1 (4) | 0.016 |
| Spitting food out by the nose | 4 (16) | 1 (4) | 0.250 |
| Spitting food out by mouth | 13 (52) | 1 (4) | 0.0001 |
| Choking with feeding | 9 (36) | 2 (8) | 0.016 |
| Need to cut food into small pieces | 14 (56) | 5 (20) | 0.004 |
| Difficulty swallowing solid foods | 8 (32) | 6 (24) | 0.500 |
| Humid breath | 9 (36) | 3 (12) | 0.031 |
| Poor weight gain | 14 (56) | 4 (16) | 0.002 |
| Hospitalization due to food aspiration | 1 (4) | 0 | 0.60 |
Comparison of Dysphagia Symptoms in Infants Before and After Intervention a
Further analyses by Fisher’s exact test were performed to determine the correlations between swallowing complications with the infant’s gender, blood culture results, and history of IVH. The results showed that 9 (47.4%) of 19 infants with a history of IVH had the symptom of choking, and IVH was the only significant risk factor for this complication before the intervention (P = 0.045). Moreover, associations between swallowing difficulties with ventilation period, gestational age, birth weight, weight at discharge, and first- and fifth-minute Apgar scores were evaluated using the independent sample t-test. The results indicated that coughing in the cases before the intervention was significantly correlated to a long ventilation period (P = 0.006), low weight at birth (P = 0.025), and discharge (P = 0.049).
Gestational age was also the only significant factor for coughing among the subjects after receiving the intervention. Gestational age was significantly lower in infants who were reported with coughing with feeding than in subjects without this swallowing complication (23 vs. 30 weeks; P = 0.030). Low weight at birth and discharge were significant factors causing spitting food by mouth before the intervention (P = 0.015 and P = 0.037). A long ventilation period could significantly result in the sensation of choking (P = 0.027). The need to cut food into small pieces was significantly correlated with low birth weight (P = 0.037) among cases before receiving any intervention (Table 3).
| Variables | P-Value | |||||
|---|---|---|---|---|---|---|
| Ventilation Period (Day) | Birth Weight (g) | Weight At Discharge (g) | Gestational Age (Week) | 1st-Minute Apgar Score | 5th-Minute Apgar Score | |
| Coughing while eating | ||||||
| Before | 0.006 | 0.025 | 0.049 | 0.085 | 0.927 | 0.781 |
| After | 0.062 | 0.197 | 0.470 | 0.030 | 0.720 | 0.620 |
| Spitting food out by the nose | ||||||
| Before | 0.503 | 0.713 | 0.959 | 0.619 | 0.202 | 0.396 |
| After | 0.450 | 0.378 | 0.450 | 0.974 | 0.199 | 0.361 |
| Spitting food out by mouth | ||||||
| Before | 0.118 | 0.015 | 0.037 | 0.135 | 0.413 | 0.459 |
| After | 0.450 | 0.378 | 0.450 | 0.974 | 0.199 | 0.361 |
| Choking with feeding | ||||||
| Before | 0.027 | 0.145 | 0.819 | 0.258 | 0.150 | 0.415 |
| After | 0.322 | 0.512 | 0.400 | 0.208 | 0.234 | 0.472 |
| Need to cut food into small pieces | ||||||
| Before | 0.396 | 0.037 | 0.130 | 0.096 | 0.988 | 0.989 |
| After | 0.847 | 0.613 | 0.247 | 0.976 | 0.107 | 0.060 |
| Difficulty in swallowing solid foods | ||||||
| Before | 0.211 | 0.415 | 0.955 | 0.995 | 0.407 | 0.566 |
| After | 0.662 | 0.586 | 0.581 | 0.595 | 0.079 | 0.329 |
| Humid breath | ||||||
| Before | 0.078 | 0.413 | 0.338 | 0.213 | 0.981 | 0.719 |
| After | 0.322 | 0.324 | 0.484 | 0.093 | 0.707 | 0.733 |
| Poor weight gain | ||||||
| Before | 0.879 | 0.964 | 0.906 | 0.698 | 0.898 | 0.479 |
| After | 0.945 | 0.120 | 0.836 | 0.432 | 0.148 | 0.062 |
Relationships Between Clinical Variables and Dysphagia Symptoms Before and After Intervention
The results also showed that after 3-6 months of interventions, dysphagia symptoms in 10 out of 24 infants (41.66%) completely and in others (38.44%) partially improved. There were also no significant relationships between the complete improvement of symptoms and underlying variables, such as ventilation period (P = 0.361), gender (P = 0.663), birth weight (P = 0.918), gestational age (P = 0.581), first- and fifth-minute Apgar scores (P = 0.592 and P = 0.083), blood culture results (P = 0.485), and history of IVH (P = 0.147).
5. Discussion
The present study was performed during the coronavirus disease 2019 (COVID-19) pandemic (2019 - 2021). Although videofluoroscopy is the gold standard for diagnosing swallowing complications (12), the virtual visit was the preferred health service during the pandemic. Therefore, the diagnosis of dysphagia among the studied participants was based on history taking and reported symptoms by the parents. Other studies showed correlations between videofluoroscopy findings and childhood dysphagia-related clinical assessment. Santos et al. indicated that the clinical evaluation of speech-language pathology had a sensitivity of 80.0%, specificity of 46.67%, a positive predictive value of 77.78%, and a negative predictive value of 77.78% for predicting dysphagia among 45 participants with cerebral palsy aged 3 - 19 years (13).
According to the results, most of the included infants (96%) had different degrees of swallowing disorders. The need to cut food into small pieces and poor weight gain were the most frequent symptoms at 56%. The aforementioned frequent rates showed that a history of a prolonged mechanical ventilation period (> 7 days) could be a significant risk factor for dysphagia. Although very few investigations reported the prevalence rate of swallowing complications among infants with a history of intubation, different prevalence rates were reported among other population studies. Da Costa et al. demonstrated that of 81 high-risk newborns, 64.2% had dysphagia (14). An investigation by Hoffmeister et al. showed that of 372 patients aged 0 - 16 years, 29% had dysphagia after extubation. The authors demonstrated that the risk of dysphagia notably increased for ages below 25 months who had prolonged intubation (15).
Comparing the above-mentioned prevalence rates shows that dysphagia in pediatric subjects, particularly those who underwent mechanical ventilation, is frequent. The undeveloped anatomy and physiology of swallowing function in infants and mechanical injuries and related comorbidities due to prolonged intubation might expose them to more significant risks of feeding complications (15). The diversity in such results might also be associated with differences in the implemented terminology to explain swallowing difficulties or dysphagia, duration of intubation, demographic characteristics, clinical assessment, and diagnostic tools, such as videofluoroscopy, history taking, and self or parents-reporting.
According to the results, a history of IVH, duration of the ventilation period, neonate’s birth weight, and weight at discharge were the significant risk factors for dysphagia. These findings are confirmed by previous investigations. Raol et al. indicated that IVH and its neurologic complications severely affect the pharyngeal phase of the swallowing and sucking reflex (16). They also pointed to mechanical intubation as another contributing factor to neonatal dysphagia (16). A positive correlation between the prolonged duration of mechanical ventilation (> 5 days) and dysphagia was also demonstrated by Brodsky et al. (17). Da Costa et al. showed significant correlations between neonatal dysphagia with neurological complications and low birth weight (< 2500 g) (14). Jadcherla also observed that neonatal feeding skills were significantly affected by birth weight (7). The author illustrated that low birth weight was a predisposing factor for neonatal dysphagia (7).
The results of the current investigation delineated that the administered interventions could entirely improve dysphagia-related symptoms in 41.66% of the participants. As any correlations could not be found between the improved symptoms and underlying variables, it is supposed that this family-based intervention significantly relieved the majority of symptoms, including coughing, spitting food out by mouth, choking, humid breath, poor weight gain, and the need to cut food into small pieces. In line with the present study’s findings, previous studies indicated the benefits of applying interventional protocols and maneuvers to improve oral feeding. Seiiedi-Biarag and Mirghafourvand, by a systematic review, showed that massage therapy could significantly decrease the frequency of vomiting and the mean gastric residual volume in 128 preterm neonates (18). Aguilar-Rodriguez et al. showed a significant improvement in oral feeding among premature neonates who received a 10-minute daily oral stimulation protocol (10). The authors concluded that such interventions could shorten the period of achieving complete oral feeding (10). Another systematic review showed that oral stimulation protocol could significantly improve oral feeding in preterm infants (19). Lau and Smith also stated the beneficial effects of sensorimotor interventions on infantile swallowing function and feeding performance (20).
The present study’s results showed that infants with a history of mechanical ventilation longer than 7 days need further attention regarding swallowing complications. Applying daily oral stimulation protocol for 3 - 6 months by parents alleviates different degrees of dysphagia.
5.1. Limitations
It should be noted that this study had several limitations. As it was conducted during the COVID-19 pandemic, some participants could not be followed and exited the study. Moreover, swallowing complications should be diagnosed using videofluoroscopy; however, due to the preference for virtual visits during the pandemic, the research tool was filling out the questionnaires. However, since the questionnaire was not validated, it imposed a limitation on the study. These explanations might indicate the reasons for the small sample size related to this study. Moreover, the IVH patients were not excluded due to the limited sample size. Further studies with larger sample sizes, different diagnostic methods, and other interventional protocols could provide further informative data.
The results of the present study delineated that infants with a history of prolonged mechanical ventilation were at greater risk of swallowing complications. The early diagnosis and implementation of sensorimotor interventions, such as oral stimulation, could alleviate different symptoms of dysphagia. Further studies with a larger sample size can provide more informative data.