1. Background
In today’s medical landscape, the use of intrapleural fibrinolytic agents (IFA) for treating empyema has become widely accepted among thoracic surgeons. Multiple studies have shown that IFA therapy offers promising results compared to surgical interventions. Several therapeutic protocols with IFAs have also been introduced, including single-agent therapy, the use of combinations of IFAs, and the employment of newer agents (1, 2). However, it is important to note that this method may not always be effective, particularly in more advanced stages of the disease. Thus, surgical intervention remains the gold standard, even after implementing strict combinational protocols. Furthermore, despite surgical decortication, empyema may not be completely resolved, or excessive manipulation of the lung parenchyma can lead to small intrapleural hemorrhages and clots. Therefore, reoperation may become unavoidable in some cases due to unexpanded lungs.
Interestingly, the instillation of IFAs through chest tubes after surgery for empyema has yet to be studied extensively. However, some studies report encouraging outcomes following intrapleural instillation of IFAs in conditions like retained hemothorax (3). Given that clots and fibrins may remain in the pleural space after thoracic interventions, the usage of IFAs postoperatively seems like a reasonable course of action.
2. Objectives
Regarding the availability and well-known adverse effects of streptokinase and alteplase, in this survey, we aimed to compare the effectiveness of these two IFAs after surgical interventions.
3. Methods
3.1. Setting and Population
This prospective cross-sectional study aimed to compare several aspects of administering two different intrapleural fibrinolytics after thoracic surgery for empyema. The survey was conducted between 2015 and 2022 in a tertiary pediatric surgery center. Every child who underwent any surgery for empyema was included in the study and prospectively followed up until the day of discharge. In the postoperative period, intrapleural fibrinolytics (streptokinase or alteplase) were injected via thoracostomy tubes for three consecutive days. Demographic characteristics and multiple variables, such as complications, length of stay, need for reoperation, etc., were obtained from the cases and compared. This study was approved by Iran National Committee for Ethics in Biomedical Research (IR.SBMU.MSP.REC.1401.654).
The diagnosis of pleural empyema was suspected based on the patient’s history, physical examination, thoracentesis cytology and biochemical evaluation, and imaging studies. Patients with prolonged symptoms (purulent cough, fever, pleuritic pain, malaise, etc.) and positive thoracentesis findings (pH < 7.2, glucose < 40 mg/dL, lactate dehydrogenase > 1000 IU/L, leukocyte count > 10000, or positive gram stain) were considered highly suspicious for pleural empyema (4). Furthermore, in equivocal cases, the diagnosis was confirmed by high-resolution computed tomography (HRCT).
3.2. Initial Management of the Disease
In confirmed cases of empyema, an age-adjusted thoracostomy tube was inserted into the pleural cavity, and empiric antibiotics were administered intravenously. Antibiotics were also changed based on culture results. In children who failed to respond to medical therapy (including preoperative IFAs), thoracic surgery was inevitable. Surgery was performed either in an open or minimally invasive manner. General anesthesia was induced in all patients using a double-lumen endotracheal tube, and the child was positioned laterally. Open surgery was performed using a posterolateral thoracotomy technique, and video-assisted thoracoscopic surgery (VATS) was carried out using three trocars and a 5-mm 30-degree endoscope. Surgical interventions included decortication and re-expansion of the affected lung. After the operation (either open or VATS), a tube thoracostomy was placed toward the lung apex posteriorly. All surgical interventions were performed under the direct supervision of board-certified pediatric surgeons. The stage of the disease was defined intraoperatively by visual inspection of the pleural cavity. These stages included stage I or exudative phase; stage II, or fibropurulent phase; and stage III, or organized phase (5).
3.3. Postoperative Care
On the first day, a chest tube was placed on continuous suction of -20 cm H2O for eight hours (6, 7). Following this, intrapleural fibrinolytic agents were administered every eight hours for three consecutive days (72 hours). The fibrinolytic agent was dissolved in 45 mL of normal saline and injected through the thoracostomy tube (8). We utilized two different fibrinolytics for this purpose: Streptokinase (20000 IU/kg) and alteplase (0.2 mg/kg). After injection, the chest tube was clamped using a nontraumatic instrument for one hour. Following the 72 hours, an imaging study (usually a plain chest X-ray) was performed to evaluate lung expansion. An HRCT scan provided further diagnostic clarity in cases with unfavorable X-ray results.
3.4. Inclusion and Exclusion Criteria
The study included children under 18 with a confirmed diagnosis of pleural empyema who underwent surgical intervention and received intrapleural fibrinolytic agents after the surgery. Patients who received IFAs preoperatively but failed in treatment were also included to compare the postoperative effects of IFAs. COVID-related empyema cases were included, but empyemas caused by nonrelated factors such as neoplasms or hydatid disease were excluded. The study did not include patients who had developed bronchopleural fistula before the surgical intervention or were missed.
3.5. Statistical Analysis
We used IBM SPSS statistics version 25 software for data analysis. Descriptive statistics were used to present demographic data, clinical features, and laboratory findings. Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range (IQR)), depending on their normality of distribution, which was evaluated using the Kolmogorov-Smirnov test. Categorical variables were presented as frequencies and percentages. We used the Mann-Whitney U test and chi-squared or Fisher’s exact test to compare the groups. A P-value of < 0.05 was considered statistically significant.
4. Results
Table 1 presents a concise overview of the preoperative findings of the study. A total of 80 patients who met the inclusion criteria were included, with only 53 receiving IFAs postoperatively. Of these, 31 (58.5%) received streptokinase, while the remaining 22 (41.5%) were in the alteplase group. Upon initial inspection, no significant statistical correlations were found between the two groups preoperative parameters. In the alteplase group, two cases had the first stage of the disease, while more third-stage cases were present in the streptokinase group (51.6% vs. 27.3%). These disparities resulted in a lower P-value (0.074), which was still insignificant.
| Variable | Streptokinase | Alteplase | Total | P-Value |
|---|---|---|---|---|
| Quantity | 31 (58.5) | 22 (41.5) | 53 | |
| Age (y) | 6.25 ± 4.8 | 6.0 ± 4.0 | 6.15 ± 4.5 | 0.840 |
| Gender | 0.561 | |||
| Female | 18 (58.1) | 11 (50) | 29 (54.7) | |
| Male | 13 (41.9) | 11 (50) | 24 (45.3) | |
| Etiology | 0.378 | |||
| Adjacent pneumonia | 29 (93.5) | 19 (86.4) | 48 (90.6) | |
| Secondary to infection, surgery, or trauma | 2 (6.5) | 3 (13.6) | 5 (9.4) | |
| Stage of empyema | 0.074 | |||
| I | 0 | 2 (9.1) | 2 (3.8) | |
| II | 15 (48.4) | 14 (63.6) | 29 (54.7) | |
| III | 16 (51.6) | 6 (27.3) | 22 (41.5) | |
| Symptoms duration (days) | 11.77 ± 6.6 | 11.18 ± 7.6 | 11.53 ± 6.9 | 0.764 |
| Symptoms | ||||
| Respiratory distress | 22 | 11 | 33 | 0.172 |
| Malaise | 16 | 11 | 27 | 0.957 |
| Fever | 29 | 17 | 46 | 0.163 |
| Pleuritic pain | 8 | 5 | 13 | 0.870 |
| Decreased lung sounds | 12 | 10 | 22 | 0.623 |
| Abdominal complaints b | 7 | 3 | 10 | 0.412 |
a Values are expressed as No. (%) or mean ± SD.
b Refers to situations like vomiting, diarrhea, abdominal pain, or ileus.
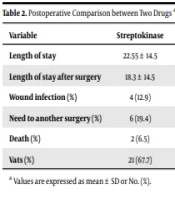
Regarding postsurgical findings, there were no significant differences in the implementation of VATS in each group (67.7% vs. 77.3%, P = 0.448). No remarkable changes were detected in the total length of stay (LOS) or LOS after surgery after using both medications. Furthermore, the mortality rate was relatively identical in the two groups. In terms of adverse complications, the incidence of wound infections was slightly higher in the streptokinase group (12.9%) compared to the alteplase group (0%), although this difference was not statistically significant (P = 0.080). Also, the study found that the need for another surgery was significantly reduced in the alteplase group (P = 0.028) (Table 2).
| Variable | Streptokinase | Alteplase | P-Value |
|---|---|---|---|
| Length of stay | 22.55 ± 14.5 | 25.36 ± 15.0 | 0.497 |
| Length of stay after surgery | 18.3 ± 14.5 | 14.9 ± 12.0 | 0.394 |
| Wound infection (%) | 4 (12.9) | 0 (0) | 0.080 |
| Need to another surgery (%) | 6 (19.4) | 0 (0) | 0.028 |
| Death (%) | 2 (6.5) | 1 (4.5) | 0.767 |
| Vats (%) | 21 (67.7) | 17 (77.3) | 0.448 |
a Values are expressed as mean ± SD or No. (%).
5. Discussion
The study found that the length of stay after surgery, wound infection, and mortality rate did not differ significantly between the groups. However, the need for another surgery due to unexpanded lungs was significantly lower in the alteplase group. These findings suggest that alteplase as an intrapleural fibrinolytic may reduce the need for reoperation after empyema surgery.
As previously discussed, the use of IFAs in managing empyema has shown promising results compared to surgical decortication alone. However, the efficacy of these agents after surgery for empyema has yet to be well-studied. Nonetheless, researchers have recommended using IFAs following postoperative retained hemothoraces (3, 9). It is worth noting that this approach has been proven safe and effective after major thoracic surgeries, such as lung transplantation (10). On the other hand, surgical procedures for empyema thoracis often require extensive and time-consuming decortication, which can result in iatrogenic intrapleural bleeding. Additionally, even with meticulous decortication and pleurectomy, fibrins and clots may remain in the pleural cavity. Consequently, the primary cause of unexpanded lungs after surgery (aside from ventilatory complications or bronchopleural fistula) appears to be retained clots and recurrent infections (11, 12). Thus, the use of IFAs, regardless of the type of surgery, could yield beneficial outcomes.
Fibrins can be naturally degraded by lytic enzymes such as streptokinase and urokinase (13). Additionally, investigators have utilized other fibrinolytic agents as IFAs (14). However, due to the absence of large-scale trials, there is no clear evidence that any single fibrinolytic agent is superior to the others in terms of outcomes. Nevertheless, when DNase is added to other fibrinolytic agents, such as tissue plasminogen activators, it can enhance fluid drainage and improve outcomes (15).
In this study, two IFAs were utilized based on their safety profiles, well-known adverse effects, prices, and availability. Alteplase and streptokinase are both fibrinolytic agents used to dissolve blood clots. When used as an intrapleural fibrinolytic, they can effectively manage complicated parapneumonic effusions and empyemas. Alteplase is a genetically engineered form of tissue plasminogen activator (t-PA). It is a highly specific fibrinolytic agent that selectively activates plasminogen bound to fibrin, leading to the dissolution of the fibrin clot. Alteplase has a short half-life, which allows for quick cessation of its effect if necessary. It has a higher fibrin specificity than streptokinase, which means it is less likely to cause bleeding complications (16). Also, streptokinase is a naturally occurring enzyme produced by Streptococcus bacteria. It binds to plasminogen and converts it to plasmin, degrading fibrin clots. Streptokinase has a longer half-life than alteplase and is less specific to fibrin, which theoretically increases the risk of bleeding complications (17, 18).
In terms of efficacy, several studies have compared the use of alteplase and streptokinase for intrapleural fibrinolysis with mixed results. Some studies have suggested that alteplase is more effective than streptokinase in achieving complete radiological resolution of the effusion and reducing the need for surgery (19). However, other studies have found no significant difference between the two agents regarding clinical outcomes (20). Regarding the existing literature, we cannot introduce the best IFA due to the lack of large trials. However, this study could show the efficacy of alteplase concerning the need for reoperation.
It should be noted that the present study had some limitations, including its relatively small sample size and the fact that it was conducted at a single center. Also, it would be better to compare our results with a control group. In addition, the decision to use IFAs and the choice of which agent to use were not randomized, which could introduce some bias into the results. Also, the prophylactic use of IFAs postoperatively can increase patient healthcare fees. So, this protocol can be useful for those parents who want an extra management protocol for their children.
5.1. Conclusions
Overall, the findings of this study suggest that the use of IFAs after surgical intervention for pleural empyema may not provide significant benefits in terms of postoperative outcomes. However, using alteplase may decrease the risk of reoperation. Further research is needed to confirm these findings and to determine the optimal treatment approach for this condition.