1. Background
Cystic echinococcosis (CE), commonly known as hydatidosis or cystic hydatid disease, is one of the most common diseases between humans and animals that cause significant economic and public health problems worldwide. Hydatidosis in many world regions, especially in parts of Latin America, Australia, Mediterranean countries, the Middle East, and India, is endemic (1-3). CE is prevalent globally in all tropical and subtropical countries, affecting 5 - 10% of the population (4). In areas where CE is common, there are 50 cases per 100,000 people yearly. Over one million people are infected with CE at any time (1-5).
Systematic reviews of cystic hydatid disease in Iran have estimated it to be 4.2 - 5% from all over the country, and the annual prevalence rate was estimated to be 0.61 per 100,000 and included one percent of all surgeries and Kermanshah in the western region with 55%; Mazandaran in North region of Iran was introduced as an endemic region with 32% of cases (6-10).
It was estimated that in Turkey, the prevalence of CE in primary school was about 0.2 - 1.1 %. According to ultrasonography reports, this prevalence was 0.59%, and over 100,000 people were infested with CE in rural areas (11-15).
This disease is caused by Echinococcus tapeworms (16). The mature worm lives in the small intestine of dogs and other carnivores as the final host, while sheep, cattle, goats, horses, camels, and humans are its intermediate hosts. Humans are unintentionally infected with parasitic eggs by consuming contaminated food, plants, and water (17). CE usually grows slowly by about 1 cm per year and is diagnosed only in 10 - 20% of patients under 16 years of age, but growth of 5 to 10 cm in one year has also been reported (18, 19). All common complications of hydatidosis are related to cyst enlargement and surgical complications (20). The liver (more than 65%), followed by the lungs (25%), is the most common location of CE in pastoral strains (21, 22). However, lung cysts are the most common type of cyst in children. Uncommonly involved parts include muscle (2 - 5%), bone (3%), kidney (2%), heart (1%), pancreas (1%), central nervous system (1%), and spleen (1%) (23-26).
In children, CE is almost asymptomatic, and the diagnosis of CE is usually made incidentally in investigation for other diseases. Lung involvement is more common in childhood, while liver involvement is more common in adults. However, any organ may be involved (27). In a study in Turkey that was done on children, 19% of patients were asymptomatic, and the main symptoms were abdominal pain (50%), fever (21.4%), and cough (19%) (5).
Human hydatidosis significantly affects public health and causes significant mortality and economic losses in the affected communities (28). The estimated direct and indirect annual costs of CE was $232.3 million in Iran and in the world is $3billion (5, 29).
Due to the slowly growing and nonspecific clinical manifestation in childhood, CE is rarely reported in children. Surveillance of childhood hydatidosis can provide useful information about the disease burden at the community level and can lead to earlier diagnosis and prevention of complications. In addition, the analysis of epidemiological factors can help better understand the disease and determine the main risk factors of human infection in each region. Although there have been some studies about hydatidosis in Iran and the world, there is little information about the prevalence of this disease in children.
2. Objectives
This study aimed to address the features and burden of this disease as basic research for interventional studies to make preventive and diagnostic strategies for reducing mortality and morbidity of children.
3. Methods
After the approval of the research plan by the research council and obtaining the code of ethics from the university's research ethics committee, this research was started. Our study population included the hospital records of children with hydatidosis in the Taleghani Children's Hospital of Gorgan from 2014 to 2021. The inclusion criteria were the records of children under 17 years of age with hydatidosis diagnosis, and the exclusion criteria were patients older than 17 years of age and incomplete files. A total of 42 children with hydatidosis whose demographic characteristics and clinical information were available were included.
Patients' demographic characteristics and clinical information, including age, gender, place of residence, history of contact with animals, serology, location of the cyst, number of cysts, type of cyst tissue, cyst size, and clinical symptoms, were recorded in the relevant forms.
The information about the location of the cyst was classified into the groups of the right liver lobe, left liver lobe, right upper, middle, and lower lobe of the right lung, and left lower and upper lobe of the left lung. Clinical symptoms were classified into two categories, including liver symptoms (RUQ pain, flank pain, abdominal pain, jaundice, and hepatomegaly) and pulmonary symptoms (shortness of breath, chronic cough, hemoptysis, chest pain, acute respiratory distress, empyema, and pleural effusion). The cyst tissue was classified into hypoechoic, solid, and mixed.
The data was collected and analyzed using SPSS software version 25. Frequencies were calculated as numbers and percentages. The difference in the prevalence of the disease based on the study's variables was investigated by the chi-square test. P-values less than 0.05 were considered statistically significant.
4. Results
A total of 58,974 children were admitted to Taleghani Children's Hospital in Gorgan during 2014-2021, of which 23,898 (40.5%) were girls and 35,076 (59.5%) were boys. Records showed that 42 (0.071%) of 58,974 hospitalized patients were diagnosed with hydatidosis. Single Liver and lung involvements separately were 13 (30.9%) and 12 (28.6%) cases, respectively, and 15 (35.7%) cases of Liver and lung were involved, one case (2.4%) in spleen and one case (2.4%) in interloop in the ileum.
The mean age of the patients was 8.5 years (range 3 - 17 years). The frequency of patients based on demographic information, including age group, gender, history of contact with animals, and place of residence, is reported in Table 1.
| Variables | No. (%) |
|---|---|
| Gender | |
| Male | 33 (78.5) |
| Female | 9 (21.5) |
| Age, y | |
| 3 - 9 | 28 (66.6) |
| 10 - 17 | 14 (33.4) |
| Residential area | |
| Urban | 11 (26.1) |
| Rural | 31 (73.9) |
| Exposure to animals | |
| Yes | 32 (76.2) |
| No | 10 (23.8) |
As shown in Table 1, two-thirds of the patients were 3 - 9 years old. According to gender, the highest frequency of hydatidosis was observed among boys (78.5%). About 76.2% of the patients had a history of close contact with animals. Also, most of them lived in villages (73.9%).
The serology test results of the subjects were 11 cases (26.2%) positive, 12 cases (28.5%) negative, and 2 cases (4.8%) unclear and because of confirmation of disease with imaging and urgent surgery; 17 cases (40.5%) did not have a serology test. A negative result was reported in 11 (91.6%) boys and one (8.4%) girl, 7 (58.5%) cases had lung involvement, 4 (33.4%) cases had liver involvement, and one (8.1%) case had liver and lung issues. The positive pathology results for CE were 22 cases (52.4%).
According to Table 2, abdominal pain in 25 patients (59.5%) and chronic cough in 19 (45.2%) were the most common liver and pulmonary symptoms, respectively. Also, fever was observed in 24 patients (57.1%) and was the second most common symptom among the patients. Other liver symptoms included hepatomegaly and jaundice. Pulmonary symptoms were shortness of breath, hemoptysis, respiratory distress, pleural effusion, and empyema.
| Variables | No. (%) |
|---|---|
| Liver cyst | |
| Abdominal pain | 25 (59.5) |
| Icterus | 1 (2.3) |
| Hepatomegaly | 5 (11.9) |
| Pulmonary cyst | |
| Chronic cough | 19 (45.2) |
| Dyspnea | 9 (21.4) |
| Chest pain | 3 (7.1) |
| Hemoptysis | 5 (11.9) |
| Respiratory distress | 5 (11.9) |
| Empyema | 1 (2.3) |
| Plural effusion | 2 (4.7) |
| Spleen cyst | |
| Left flank pain | 1 (2.3) |
| Other presentations | |
| Fever | 24 (57.1) |
| Convulsion | 1 (2.3) |
| Low level of consciousness | 1 (2.3) |
| Asymptomatic | 5 (11.9) |
In the involvement of the spleen, left flank pain was reported in one case (2.3%). Other symptoms included convulsions in one case (2.3%) and decreased level of consciousness in another case (2.3%), and five patients (11.9%) were asymptomatic.
In the current study, patients were divided into four groups based on the location of cysts, including patients with lung cysts, liver cysts, and spleen and abdominal cysts. Surgery was performed in all patients with lung cysts. Fifteen (55.5%) of 27 patients with liver cysts, one patient (100%) had a spleen mass, and 2 of the patients (100%) had an abdominal mass. Two patients with liver cysts did not personally consent to surgery, and one case had a COVID-19 infection. Others were treated with albendazole.
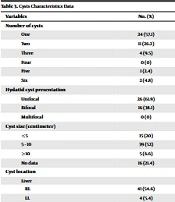
The number of cysts in our subjects ranged from 1 to 6 cysts. The frequency distribution of the number of cysts is reported in Table 3. Unifocal cysts were observed in 26 cases (61.9%), and bifocal cysts or involvement of two organs in 16 cases (38.1%). However, our study subjects had no multifocal cyst or multi-organ involvement (Table 3).
| Variables | No. (%) |
|---|---|
| Number of cysts | |
| One | 24 (57.1) |
| Two | 11 (26.2) |
| Three | 4 (9.5) |
| Four | 0 (0) |
| Five | 1 (2.4) |
| Six | 2 (4.8) |
| Hydatid cyst presentation | |
| Unifocal | 26 (61.9) |
| Bifocal | 16 (38.1) |
| Multifocal | 0 (0) |
| Cyst size (cm) | |
| ≤ 5 | 15 (20) |
| 5 - 10 | 39 (52) |
| ≥ 10 | 5 (6.6) |
| No data | 16 (21.4) |
| Cyst location | |
| Liver | |
| RL | 41 (54.6) |
| LL | 4 (5.4) |
| Total | 45 (60) |
| Pulmonary | |
| RUL | 2 (2.6) |
| RML | 6 (8) |
| RLL | 13 (17.4) |
| LUL | 1 (1.4) |
| LLL | 5 (6.6) |
| Total | 27 (36) |
| Spleen | 1 (1.4) |
| Abdomen | 2 (2.6) |
| Cyst type | |
| CE1 | 30 (40) |
| CE2 | 6 (8) |
| CE3a | 13 (17.4) |
| CE3b | 0 (0) |
| CE4 | 0 (0) |
| CE5 | (0) |
| No data | 26 (34.6) |
| Kind of cyst | |
| Hypo echo | 36 (48) |
| Solid | 0 (0) |
| Mixed | 27 (36) |
| No data | 12 (16) |
Abbreviations: RL, right lobe; LL, left lobe; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
The size of the cysts in our patients varied from ≤5 cm to ≥10 cm, and those with a size of 5-10 cm were the most frequent, although the size of the cyst in 16 cases (21.4 %) was not recorded.
According to Table 3, the most common location of the cyst was the liver, with 45 cases (60%), followed by the lung, with 27 cases (36%). The least involvement of the abdominal mass and spleen was observed in 2 cases, respectively, and no mass was recorded in the brain. In liver cysts, the right lobe was the most common location, with a frequency of 41 cases (54.6%). Involvement of the left lobe of the liver was reported in 4 patients (5.4%). In lung cysts, the RLL of the lung was the most common location of the cyst, with a frequency of 13 cases (17.4%) (Table 3).
As shown in Table 3, according to the WHO classification, the type of cysts in order of frequency, 30, 13, and 6 cases were CE1, CE3a, and CE2, respectively. The frequency of CE3b, CE4, and CE5 was zero, and there was no relevant information in 26 cases (34.6%).
There were 36 hypoechoic cysts and 27 mixed cysts, but the frequency of solid cysts was zero. (Table 3)
According to Table 4, the risk of cystic hydatid disease in boys was 2.5 times higher in girls, and rural areas were three times more frequent than urban areas.
| Variables | P-Value | OR | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| Gender: Female (ref)/male | 0.015 | 12.4982 | 1.1952 | 5.2216 |
| Location area: City (ref)/village | 0.001 | 13.2190 | 1.6182 | 6.4048 |
5. Discussion
Hydatidosis is an important public health issue in children in terms of prevalence and characteristics. CE infests 29600 people annually, causing 17000 deaths and costing USD 3 billion (30). The World Health Organization considered Iran a hyperendemic region (31). Due to the lack of information about CE in children and the necessity of prompt diagnosis for preventing morbidity and mortality, this study investigated the clinical findings of CE in children admitted to Taleghani Hospital in Gorgan from 2014 to 2021.
The average age in our study was 8.5 years (age range 3 - 17 years), which, although the age spectrum was similar in most studies, compared to the studies of Sarkari (6.8 ± 3.7) and Aslanabadi (7.93 ± 3.0), it was higher. Compared to the study of Sanaei Dashti (11.5 ± 6.1) and Ozdemir (9.8), Tartar (10.3 ± 2.9), Fahimzad (9.25 ± 3.37), Vlad (10.8) and Akgul Ozmen and Onat (9.6 ± 3.9) however, our cases were younger (22, 32-38).
In this study, it was found that 66.6% of the patients belonged to the age group of 3 - 9 years, and similar to Sarkari et al.’s and Fahimzad et al.’s studies, 68.6 % and 61.1% of patients were younger than 10, respectively (32, 36). The high prevalence of CE in children younger than 10 when they play with soil and animals indicates that we should promote awareness of this disease and the route of infestation to prevent CE in early childhood.
Regarding gender, the highest prevalence of CE in the present study was observed among boys at 78.5%, and this difference between genders was statistically significant based on the chi-square test (P = 0.012). Similarly, most studies have demonstrated that boys are more likely to suffer from CE (22, 33, 35). In Mishra's study, boys were 3 times more likely to be infected than girls (39). In another study, however, the difference in statistics between boys and girls was almost negligible (32, 40, 41), but some studies reported that CE was more common in females (6, 34). In Moosazadeh’s study, this rate was higher in women, but the study was conducted in adult patients, and this difference may be attributed to the age of the participants (31).
This study showed that 72% of the patients had a history of contact with animals. Similar to our results, in other studies, the rate of contact with animals was reported between 59.5% and 68.4% (32, 34, 42). The high rate of close contact in this study highlighted that one of the preventive strategies for CE in this region is to keep children away from untreated animals and refrain from keeping animals indoors. The animals kept near families should be regularly visited and treated by a vet.
In the present study, 73.9% of patients lived in villages, which was in line with previous studies (63.1 to 83%) (34, 42). Although in this study, the rate of patients with CE in rural areas was three times higher than in urban areas, we should inform the local health organizations about the burden of CE in both urban and rural regions to plan educational programs for awareness among local people about the preventive strategies to control CE locally. All organizations should be involved in reducing and controlling CE to save children's lives.
According to National Parasitology Reference Laboratories, serum samples of suspected CE cases were reported as 16.2% positive in Turkey, and this figure was 37.6 - 42.9% in other studies in Turkey (3, 43, 44). In this study, ignoring the cases where the test results were unclear, more than half of the results of serological tests were reported as negative. The negative test was higher in boys (91.6%) than in girls, and 91.9% reported only liver or lung cysts. In a Turkish study, 36.4% tested positive for hepatic cysts and 75% for pulmonary cysts; in our study, the percentages were 46% and 66%, respectively (3). This suggests that pulmonary cysts are associated with more positive serology results than liver cysts. On the other hand, false negative report in males needs additional research to evaluate the possible sex effect on the laboratory results. Other reasons may be the serology method, the rate of contamination in children, the early stages of the cyst disease, the size of the cysts that were still very small, or lack of information on patients' files (45-48). Although negative serology results in CE do not indicate a definite diagnosis, it is suggested that more studies should be conducted to find a valuable serum marker with proper sensitivity and specificity to protect children from imaging radiation.
According to the conducted studies, most surgeons tend to manage CE without intervention and with "watch and wait," or drug treatment instead of surgical treatment, and only complicated cases undergo surgery, which leads to a decrease in the number of patients who undergo surgery (40). In the present study, hydatidosis surgery was performed in 100% of patients with lung cysts, 55.5% with liver cysts, and 100% with spleen and abdominal masses. Some of the reasons for the low rate of surgery in our study were lack of personal consent to perform surgery in two patients and covid19 infection in one of the study subjects. Previous studies have reported different surgery rates. Other studies reported the surgery rate as 60.5 - 100% (22, 34, 36, 39, 41, 42).
In the present study, one single cyst was the most frequent type, with a rate of 57.1%, similar to other studies (68.1 - 76.3%) (39-41, 48). Unifocal cysts were observed in 61.9%, and bifocal cysts in 38.1%. However, there was no multifocal cyst or multi-organ involvement in our study subjects, consistent with previous reports that ranged between 53.5 - 59.6% (34, 36, 42). It is recommended that when a cyst is reported in the lung or liver, other involvements should be investigated by sonography or chest X-ray.
Hydatidosis presents with different symptoms based on the site of involvement, the size and number of cysts, the patient's age, and sometimes asymptomatic. Chest pain, cough, and hemoptysis are the most common symptoms in the CE of the lung. The symptoms of liver hydatidosis have been reported in previous studies to be very different and related to their location, type, and complications. Consistent with previous studies, cough was the most common symptom of lung cysts in the present study. According to other studies, the prevalence of coughing ranged from 47 to 100% (22, 34, 35, 39).
The size of cysts in the human body is different and is usually between 1 and 15 cm, but much larger cysts (diameter 20 cm) may also form. Hydatidosis in our patients was very different in terms of the size of the cyst, but cysts ranging in size from 5 - 10 cm had the highest frequency at 52%, which is consistent with other studies (34, 36).
According to the nature of portal blood flow, most of the cysts are localized in the liver and lung's right lobe, similar to the previous studies (36). The quality of Cystic hydatid in our study was 48% hypoechoic, 36% mixed, and zero solid, respectively, and the type of cyst was not reported in 16%. Our findings were similar to the report of Sanaei Dashti et al. in this study. A hypoechoic cyst was reported in 47.4%, a mixed cyst in 28.1%, and a solid cyst in 1.75%, and the type of cyst was not reported in 22.8% (34).
This study has limitations, including retrospective design, small sample size, and incomplete documentation. Possible reasons for the lower number of patients, as compared to previous studies, include an increase in the general level of health and awareness of health issues among people, better management of slaughterhouses, a reduction in the number of stray dogs, and a shift away from traditional animal husbandry practices compared to previous years. The differences observed in the present study and other studies may be due to the difference in sample size, patient age, study area, disease management and treatment techniques, and patient follow-up time.
According to this study, the prevalence of CE in Golestan province is high, and children, especially the male gender and those living in rural areas are more susceptible to the disease. It is recommended that local health organizations pay attention to this issue and hold educational programs to promote knowledge about this disease and infestation prevention in rural and urban areas. On the other hand, physicians in this region should be educated about the signs and symptoms of hydatidosis to diagnose and treat patients promptly. Proper serum markers should be introduced for early diagnosis, and more research should be conducted on this disease.
5.1. Conclusions
Hydatidosis is one of the most important human cestode infections, and in this study, its prevalence was higher among boys aged 3-9 in rural areas. The liver and lung involvement is common in children, which requires surgery in most cases. Considering the high prevalence of this disease in our region, it is necessary to design an intervention plan to reduce children's burden, and a multidisciplinary approach should be planned to reduce the mortality and morbidity of children.