1. Background
In recent decades, therapeutic plasma exchange (TPE) has become a well-established treatment method for various pediatric neuroimmunological disorders. Its effectiveness lies in removing circulating autoantibodies, immune complexes, and cytokines from the blood, followed by volume replacement with fluid. In summary, TPE involves drawing venous blood into an external circuit, separating the cellular components from the plasma, and replacing the plasma with fresh frozen plasma and albumin (1). The first instance of TPE dates back to 1960 when Schwab and Fahey performed the procedure to reduce high globulin levels in a patient with macroglobulinemia (2).
The American Apheresis Society strongly advocates for plasma exchange as a primary treatment option in diseases like Guillain-Barré syndrome (GBS) and N-methyl-D-aspartate receptor (NMDAR)-related autoimmune encephalitis and as a secondary treatment option in diseases like acute disseminated encephalomyelitis (ADEM), multiple sclerosis, neuromyelitis Optica spectrum disorder (NMOSD), and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) (3).
The efficacy of TPE in various pediatric neuroimmunological disorders has been demonstrated in the existing literature (4-7). Recently, Akcay et al. (8) evaluated the effectiveness of TPE in patients with multiple sclerosis (MS), transverse myelitis (TM), and ADEM, using EDSS and GS scoring criteria. They reported positive outcomes with this treatment method. Similarly, Atay et al. (9) studied 20 patients with neuroimmunological disorders, including autoimmune encephalitis (AE), GBS, and ADEM, and observed clinical improvement after TPE.
2. Objectives
In our current study, we present our experience with TPE in children with neurological disorders at a single center and describe clinical responses.
3. Methods
In this cross-sectional study, we investigated patients under 18 diagnosed with neuroimmunological diseases, including optic neuritis (ON), MS, NMOSD, GBS, ADEM, and AE. The study focused on patients who underwent therapeutic plasma exchange at the neurological department of Mofid Children’s Hospital in Iran over one year from March 2021.
The inclusion criteria for this study consisted of neuroimmunological patients who, despite receiving first-line treatments, obtained inadequate treatment response or, due to the severity of the disease and its rapid progression, required plasma exchange for their treatment. Furthermore, patients were excluded from the study if they died during plasma exchange due to reasons unrelated to the procedure itself or if their diagnosed condition changed to non-neuroimmunological disorders after completion of the procedure. The research did not include patients with primary autonomic and coagulation disorders.
The MS diagnosis was based on McDonald’s criteria, and NMOSD was diagnosed based on clinical symptoms, radiological findings, and anti-aquaporin-4 antibodies. Also, GBS diagnosis relied on clinical signs and confirmation through electromyography/nerve conduction velocity tests, while ADEM diagnosis was established based on clinical symptoms and radiological findings. Finally, autoimmune encephalitis diagnosis followed the criteria for encephalitis and the presence of antibodies against GAD, NMDA, and other receptors.
A pre-designed checklist was used to record demographic information, side effects experienced after plasma exchange therapy, and the clinical effectiveness of the treatment. Clinical examinations specific to each disease were conducted before and after each cycle, and the effectiveness of the treatment was evaluated during the 3 to 6 months follow-up period after discharge based on clinical examination improvement. The plasma exchange procedure was performed using apheresis therapy equipment (Spectra Optia Apheresis 61000 model). The patients received anesthesia, and the surgical team inserted a double-lumen catheter. Prior to each procedure, routine tests including complete blood count (CBC), calcium (Ca), phosphate (Ph), magnesium (Mg), prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), blood urea nitrogen (BUN), and creatinine (Cr) were conducted, with subsequent rechecks after each cycle to monitor any changes.
In each therapeutic plasma exchange cycle, 1 to 1.5 times the patient’s plasma volume was replaced daily. Plasma fluid substitutes such as 5% albumin and fresh frozen plasma were used at a ratio of 4 to 1. Calcium gluconate was administered at a rate of 1 cc per kilogram of body weight, up to a maximum of 10 cc per cycle, to prevent hypocalcemia. For children weighing 20 kg or less, 1 unit of packed red blood cells (250 cc) was administered at the start of the cycle to prevent hemodynamic disorders. The number of plasma exchange cycles for each patient ranged from 5 to 10, depending on the disease and its severity.
Pediatric neurologists utilized the Modified Rankine Scale (MRS) to retrospectively assess disability based on clinical examination data (7, 10, 11). Disability levels were evaluated at three different time points: (1) prior to commencing therapeutic plasma exchange, (2) immediately after completing the plasma exchange cycles, and (3) during the 3 to 6 months follow-up period. The extent of improvement was determined by the reduction in the MRS score. A one-point decrease in the score was categorized as a mild improvement, a two-point decrease as a moderate improvement, and a decrease of three or more points as a significant improvement. Complete recovery was defined as the absence of mental, motor, and social complaints reported by either the parents or the child during the follow-up period. Statistical analysis was conducted using SPSS software (version 28) (SPSS Inc., Chicago, IL). Descriptive statistics and standard deviation were employed for the analysis.
4. Results
4.1. Demographic Findings
Out of 20 patients who underwent plasma exchange at Mofid Children’s Hospital in Iran within one year starting from March 2021, 18 patients were included in the study. Two patients were excluded from the study due to a change in their final diagnosis. One patient was diagnosed with progressive neurodegenerative disease; the other had neurobehçet syndrome. The initial diagnoses of the included patients were as follows: NMOSD in 4 patients (22%), AE in 4 patients (22%), MS in 4 patients (22%), ADEM in 1 patient (6%), GBS in 4 patients (22%), and ON in 1 patient (6%).
Among all the patients, four (22%) received intravenous immunoglobulin (IVIG) prior to plasmapheresis, eight patients (44%) received steroids, and five patients (28%) received both IVIG and steroids before undergoing plasmapheresis. One patient (6%) received no treatment before plasma exchange.
A total of 121 procedures were performed on the included patients. The oldest patient in the study was 15 years old, and the youngest patient was four years old. The highest average weight was observed among the MS patients, while the lowest average weight was seen in the GBS group (Table 1).
| Diagnosis | Case, No. | Age | Age Ave. | Age SD | Weight (Kg) | Weight Ave. (Kg) | Weight SD | Gender | Initial Treatment | Number of TPE Procedures |
|---|---|---|---|---|---|---|---|---|---|---|
| MS | 4 | 14 | 12.50 | 1.12 | 73 | 53.25 | 13.46 | Female | Steroid | 8 |
| 13 | 13 | 52 | Female | Steroid | 7 | |||||
| 14 | 12 | 53 | Male | Steroid | 8 | |||||
| 18 | 11 | 35 | Female | Steroid | 5 | |||||
| NMOSD | 5 | 11 | 9.50 | 1.50 | 34 | 30.75 | 8.01 | Female | Steroid | 7 |
| 6 | 7 | 19 | Female | IVIG/steroid | 5 | |||||
| 11 | 10 | 41 | Male | Non | 7 | |||||
| 15 | 10 | 29 | Female | IVIG/steroid | 7 | |||||
| AE | 1 | 12 | 12.50 | 1.12 | 60 | 45.25 | 8.53 | Male | IVIG | 10 |
| 9 | 11 | 40 | Male | IVIG/steroid | 5 | |||||
| 16 | 14 | 41 | Male | Steroid | 7 | |||||
| 17 | 13 | 40 | Female | Steroid | 8 | |||||
| GBS | 3 | 9 | 8.75 | 3.19 | 24 | 27.00 | 13.87 | Male | IVIG | 7 |
| 7 | 4 | 13 | Female | IVIG | 5 | |||||
| 8 | 9 | 21 | Male | IVIG/steroid | 5 | |||||
| 10 | 13 | 50 | Male | IVIG | 7 | |||||
| ADEM | 2 | 15 | 15 | - | 50 | 50 | - | Male | IVIG/steroid | 8 |
| ON | 12 | 12 | 12 | - | 47 | 47 | - | Female | Steroid | 5 |
Abbreviation: SD, standard deviation.
4.2. Effectiveness
4.2.1. Multiple Sclerosis Subgroup
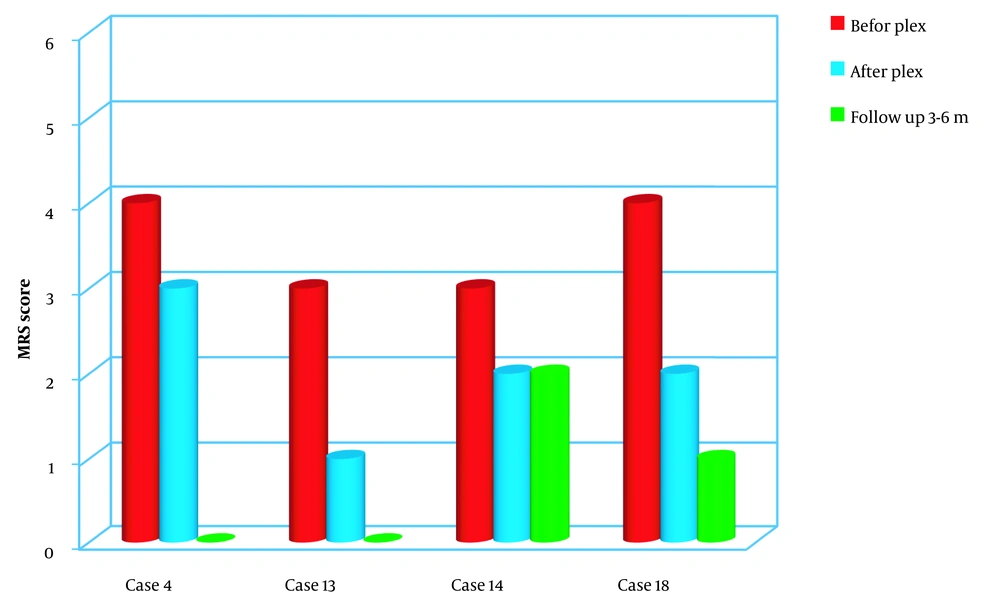
Out of four MS patients studied, two presented solely motor symptoms, one had a combination of motor symptoms and ocular involvement, and one had only ocular manifestations. All four patients demonstrated positive changes in their examinations immediately after undergoing plasmapheresis.
During the follow-up period, the patient with ocular manifestations maintained unchanged visual acuity in the left eye while experiencing a decrease of one-tenth in visual acuity in the right eye. However, significant improvement was observed in the motor symptoms of the two patients compared to their condition prior to plasma exchange (Figure 1).
4.2.2. Neuromyelitis Optica Spectrum Disorder Subgroup
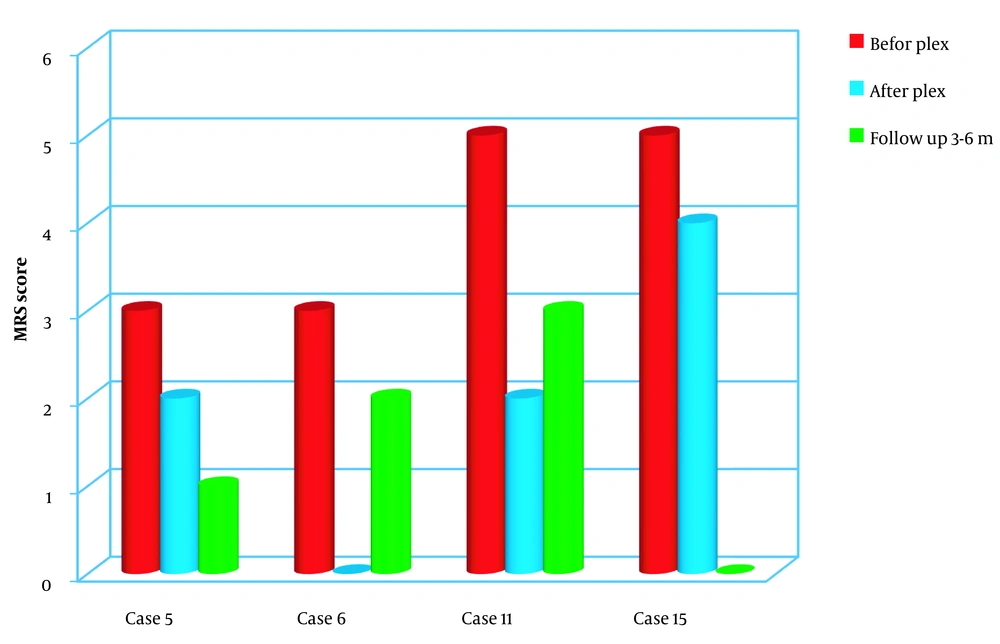
In the group of NMOSD patients, there was a diverse range of presentations. One patient had motor manifestations, another exhibited reduced consciousness and visual changes, and two presented solely with ocular manifestations. Following plasma exchange, all four patients showed positive changes in their examinations.
Although two patients in this group experienced improvements in their eye conditions, the degree of improvement was not significant compared to the improvement observed in motor symptoms. During the follow-up period, one patient showed considerable improvement, one demonstrated moderate improvement, and two experienced the recurrence of visual symptoms (Figure 2).
4.2.3. Autoimmune Encephalitis Subgroup
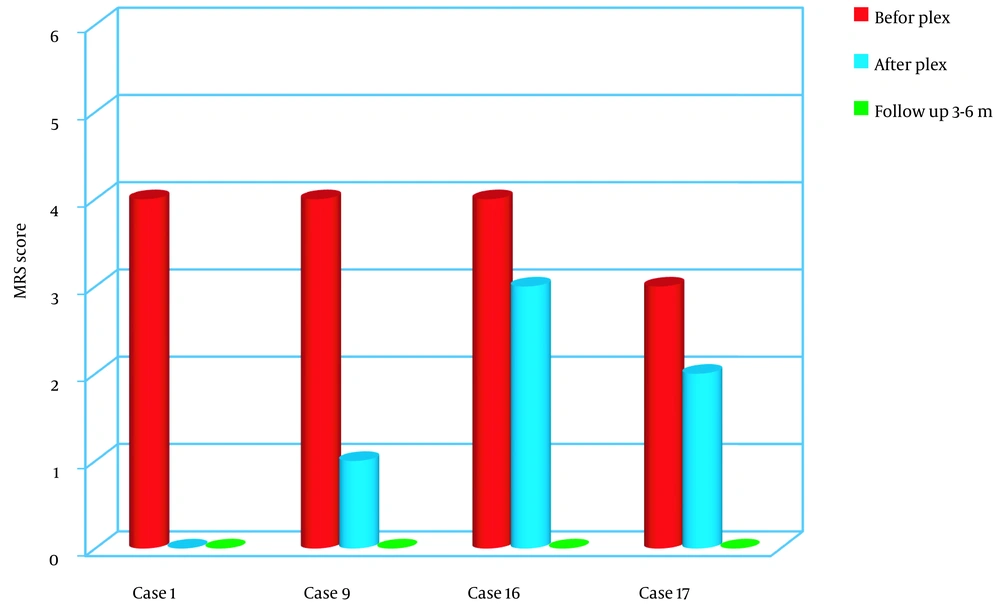
The AE group exhibited the most favorable outcomes in this study. All four patients showed significant improvements when comparing the patients’ conditions before and after plasmapheresis. Specifically, when comparing the pre-TPE and post-TPE assessments, all four patients achieved a Glasgow Coma Scale (GCS) score of at least 14 out of 15.
Among the two patients who initially presented with seizures and behavioral changes in addition to the loss of consciousness, plasmapheresis successfully controlled the seizures, and the behavioral changes were completely resolved. In the other two patients, behavioral changes persisted as behavioral slowness, but these symptoms eventually normalized during the follow-up period.
During the examination conducted 3 to 6 months later, all neurological assessments fell within the normal range, demonstrating significant improvement compared to the pre-plasma exchange condition (P < 0.05) (Figure 3).
4.2.4. Guillain-Barré Syndrome Subgroup
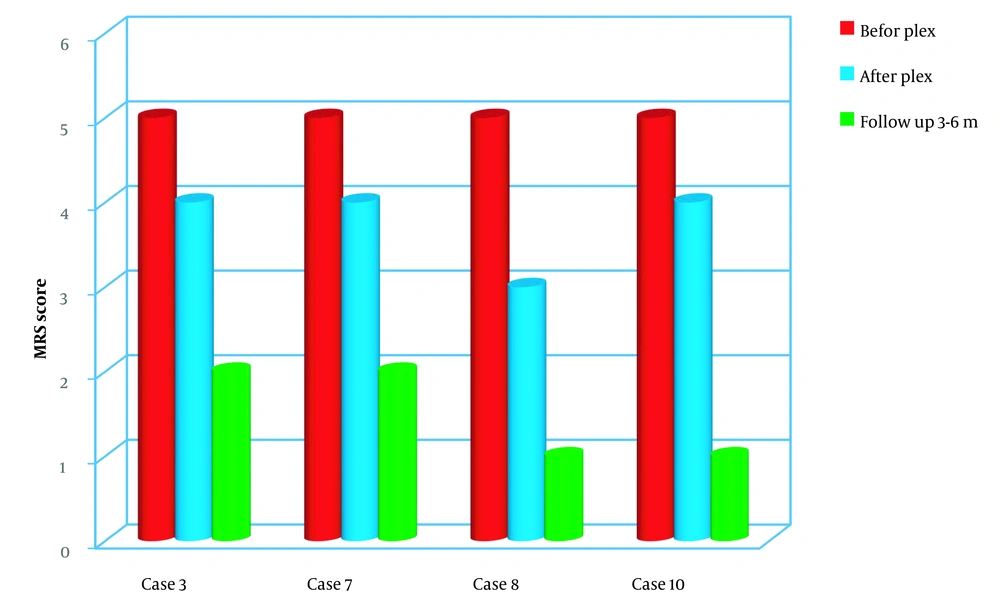
Among GBS patients, three out of four were diagnosed with acute motor axonal neuropathy (AMAN), while one had acute motor and sensory axonal neuropathy (AMSAN). Before plasmapheresis, three of the four patients did not exhibit a gag reflex. As a result, all three patients with absent gag reflexes were admitted to the PICU due to respiratory muscle weakness, and one of them required intubation.
After undergoing plasmapheresis, all four patients showed improvements in neurological examinations and symptomatology. During the follow-up period, significant improvements were observed in all four patients, indicating a statistically significant difference (P < 0.05) (Figure 4).
4.2.5. Acute Disseminated Encephalomyelitis Subgroup
In this subgroup, a single patient was studied. The patient was referred due to seizures, decreased consciousness, and changes observed in brain and spinal cord MRI scans. Therapeutic plasma exchange was performed for this patient as an intervention when the initial treatment, including steroids and IVIG, did not yield a satisfactory response.
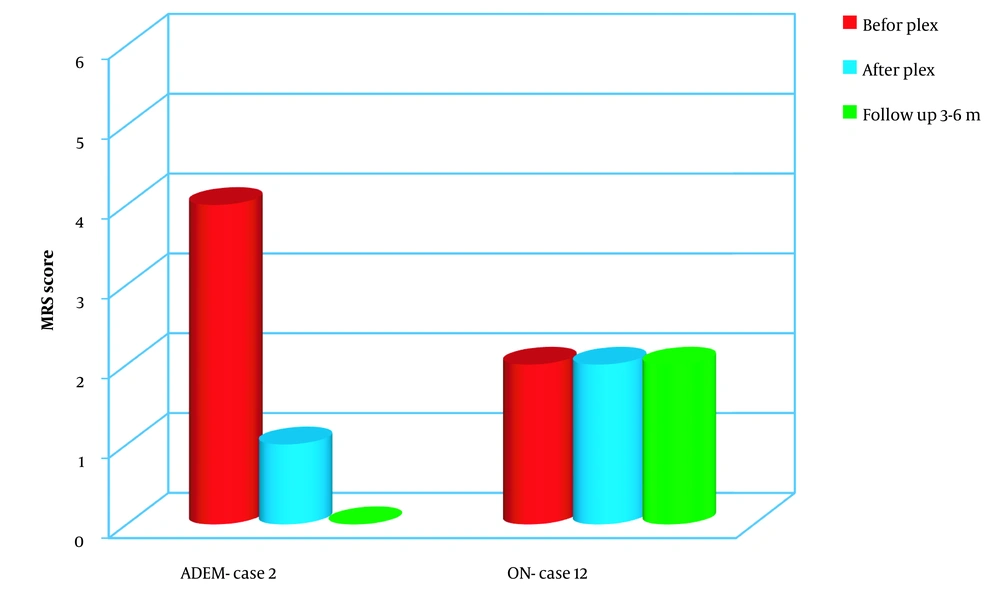
The patient showed notable improvements in symptomatology and seizure control after the plasma exchange procedure. During the follow-up period, all neurological examinations conducted on the patient fell within the normal range, indicating significant improvement (Figure 5).
4.2.6. Optic Neuritis Subgroup
In the patient with optic neuritis included in the study, the individual presented with severe vision loss in the right eye, equivalent to one-tenth of normal vision, along with optic nerve atrophy. Despite undergoing five cycles of plasmapheresis, no improvement was observed in the patient’s vision. Furthermore, during the follow-up period, no changes in the patient’s condition were observed (Figure 5).
4.3. Side Effects
A comparison was conducted between the laboratory findings before and after plasma exchange in the 18 patients included in the study. No complications were observed that would pose problems for the patients. In a patient within the MS subgroup, the initial platelet count prior to therapeutic plasma exchange was 294,000/μL, which decreased to 110,000/μL after the TPE procedure. Considering the absence of any significant symptoms in the patient and a platelet count above 100000/μL, no specific therapeutic intervention was required during hospitalization. Additionally, the platelet count returned to the normal range (203,000/μL) during the 3-month follow-up period. Furthermore, out of the 121 plasma exchange cycles performed, 11 cycles (9.09%) had mild complications. It is worth noting that none of the patients experienced adverse effects during the subsequent follow-ups.
5. Discussion
In recent years, the TPE method has been increasingly used with promising results in treating children with various diseases (12). Specifically, TPE has been increasingly utilized in treating autoimmune neurodegenerative disorders, aligning with international guidelines that recommend early administration of TPE in severe cases of pediatric autoimmune encephalitis (13). Previous studies have demonstrated response rates to TPE in autoimmune encephalitis ranging from 47% to 85% (3, 4). In their study following TPE, Atay et al. reported mild improvement in 5 out of 20 patients diagnosed with autoimmune encephalitis (9). However, in our present study, all four patients with autoimmune encephalitis exhibited complete recovery after TPE treatment. Manguinao et al. (14) observed symptom improvement in 23 out of 26 patients with acute central nervous system demyelinating disorders who underwent TPE.
Additionally, all 15 patients with MS and five out of seven with NMOSD exhibited clinical improvement (14). Similarly, in our study, out of 18 patients, 17 demonstrated improvements in clinical symptoms following TPE. Among the MS subgroup, three out of four patients exhibited apparent improvement, while one showed partial improvement that was not as significant as the others. Among the four patients in the NMOSD subgroup, two with visual impairment experienced mild improvement compared to the other two. Kumavat et al. also demonstrated the effective management of severe acute attacks of NMOSD through TPE (15). Clinical data from 30 adult patients indicated moderate or significant improvement (73.3%), with TPE frequently employed as the primary treatment for NMOSD.
Furthermore, a review article by Kosiyakul et al. revealed a satisfactory response to TPE in over 60% of patients with positive aquaporin-4 (AQP4) antibodies (16). The study further noted that NMOSD patients with positive AQP4 antibodies and reduced vision exhibited a weaker response to treatment, higher relapse rates, and an increased risk of treatment failure (17). In our study, two patients experienced a recurrence of eye symptoms during follow-up, with one testing positive for AQP4 antibodies, which can be attributed to this observation. In the present study, both MS and NMOSD patients underwent a rituximab maintenance regimen following acute treatment, and the observed improvement in some of these patients during follow-up may be attributed to the positive effects of this treatment.
As known, TPE and IVIg are the most effective treatments for GBS in both children and adults, surpassing conservative therapy in terms of improving disability (18-20). Most previous studies have found no significant differences between pediatric and adult GBS patients regarding TPE efficacy, treatment response, and neurological outcomes (19-23). However, a few studies have reported that TPE is more effective than IVIg in mechanically ventilated children with GBS (24). Also, TPE is most beneficial when initiated within the first four weeks of disease onset (22). In the present study, TPE was initiated within 10 days for three out of four patients, highlighting the importance of early administration of TPE and the satisfactory response observed in these patients.
Notably, isolated ON is rare in children and is typically associated with demyelinating diseases such as MS and NMOSD (25). In our study, we investigated a patient diagnosed with isolated ON. Despite undergoing TPE for the affected eye, which exhibited one-tenth vision and optic nerve atrophy, no improvement was observed. However, the symptoms resolved in the opposite eye, with 9/10 vision and mild pain. Previous studies, including Deschamps et al. (26), demonstrated improvement in over 50% of adult patients diagnosed with ON following TPE. Moreover, when ON was present on the same side, TPE was significantly associated with poor treatment response. In the case of our patient, the late referral for treatment and severe atrophy of the right optic nerve can be considered acceptable explanations for the lack of response to TPE. Although TPE did not impact the affected eye, it likely prevented the regression of visual acuity in the other eye.
The present study observed positive changes in patients with NMOSD and MS at two stages: After plasma exchange and during the follow-up period of 3 to 6 months post-treatment. Particularly, patients with motor manifestations demonstrated greater improvement than those with solely ocular manifestations or ocular-motor manifestations. The least changes in the MRS score in the follow-up were observed in two groups of patients with MS and NMOSD who presented with concurrent visual and motor impairments as their initial complaint.
5.1. Conclusions
In conclusion, this study demonstrated that TPE is an effective treatment option with minimal side effects for children with neurodegenerative diseases caused by antibodies, particularly GBS and AE. The findings support using TPE as a beneficial therapeutic approach in these patient populations. It is recommended that future research should encompass comprehensive studies within each specific group of pediatric patients to further investigate the potential benefits and outcomes of TPE. By expanding the scope of research, we can gain a deeper understanding of the efficacy and safety of TPE in different neurodegenerative diseases in children.