1. Background
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first determined in December 2019. COVID-19 was less frequent in children compared to adults at the start of the pandemic (1 - 5%). Since April 2020, many studies have shown that COVID-19 could lead to a childhood hyperinflammatory syndrome in which symptoms are like those of Kawasaki disease (1-6). Multisystem inflammatory syndrome in children (MIS-C) conducted to SARS-CoV-2 was recently defined by the World Health Organization (WHO) and the Centres for Disease Control and Prevention (CDC) (7, 8).
Clinical symptoms and imaging modalities about myocardial dysfunction and coronary involvement related to MIS-C were mentioned in many articles (2-4, 9, 10). Electrocardiogram (ECG) evaluation with the association of biomarkers could be important prognostic markers in the assessment of prognosis.
Although ECG findings could have important impacts on the prognosis of the disease, there is very limited data on evaluating the ECG of these patients. In a recent study including the ECGs of MIS-C patients, dysrhythmia incidence was discovered to be 21% (6). The incidence of arrhythmia in MIS-C was 12% in another cohort study, but detailed information about the risk factors for dysrhythmia was not established (11). There were only a few cases regarding dysrhythmia assessment in MIS-C.
Although ventricular repolarization alterations and associated arrhythmia were defined in Kawasaki disease in earlier studies, to the best of our knowledge, no study concerning cardiac arrhythmia risk evaluation in MIS-C has been published (12).
2. Objectives
This study aimed to analyze the ventricular repolarization properties in MIS-C and to examine the cardiac arrhythmia probability in these patients.
3. Methods
3.1. Study Populations
This prospective, cross-sectional, controlled study was carried out between January 27, 2022, and January 1, 2023, by the Paediatric Cardiology and Paediatric Infectious Disease departments of the Mersin University Hospital. The MIS-C diagnosis was made based on the WHO and CDC criteria (7, 8). The cardiovascular physical examination of all children was recorded. The biochemical data (leukocytes, hemoglobin, aspartate aminotransferase, alanine aminotransferase, albumin, C-reactive protein (CRP), procalcitonin (PCT), sedimentation, D-dimer), and cardiac markers (N-terminal pro-brain-natriuretic peptide (NT-proBNP), troponin I, creatine kinase (CK), and creatine kinase-myocardial band (CK-MB) were checked in the patient group.
At the beginning, all ECGs were analyzed. The patients with MIS-C were divided into 2 groups, mild MIS-C (n = 15) and severe MIS-C (n = 14), according to the established criteria (13). Some patients with a more serious clinical course were evaluated in the severe form according to the predominance and severity of cardiac functions, cardiac enzyme levels, BNP values, accompanying hypotension, vasoactive treatment requirement, respiratory support, arrhythmias, and noncardiovascular gastrointestinal system involvement. Mild cases did not need any vasoactive requirement with minimal/no respiratory support and minimal organ injury, while severe cases needed inotropic vasoactive treatment associated with non-invasive or invasive ventilatory support and severe organ injury, including moderate-to-severe ventricular dysfunction. Electrocardiogram data for 82 age- and sex-matched healthy children were compared to those of the sick ones. These 82 healthy children in the control group visited our clinic because of palpitation, heart murmur, or chest pain, and had no cardiac pathologies, including structural and rhythm problems, and took no medications. The ECG parameters of all groups were calculated. The inclusion criteria for the control group were that they could have no other cardiologic diseases, including structural heart diseases and arrhythmia syndromes. The exclusion criteria for patients and the control group were that they have other systemic diseases. Therefore, children with chronic illnesses and those taking daily medications were excluded. There were no arrhythmic and/or antiarrhythmic drug use, no history of metabolic diseases, and no significant electrolyte disturbances that would make a significant difference in the ECG parameters of the patient and control groups. The local Ethics Committee accepted the protocol.
3.2. Electrocardiography
Standard 12-lead ECG was done for the patients and the controls at a paper speed of 25 mm/second, all of whom have similar conditions. A Nihon Kohden ECG 1250 Cardio fax S (2009, Tokyo, Japan) device was utilized with standard settings. Electrocardiography frames had a good resolution, and measurements were made on a computer by 2 experienced pediatric cardiologists. Duration of QT intervals was identified in all derivations, and average scores were recorded, in which the beginning of the QRS complex and the ending of the T wave to baseline were ascertained. The QT duration was arranged for the heart rate based on Bazett's formula to assess corrected QT (QTc). Heart rate, T peak-end (Tp-e), Tp-e dispersion, and Tp-e/QT ratio were also adjusted. Tp Te was calculated via the tangent method in precordial leads (14, 15). A tangential line was dragged to the downward curve of the T wave and crossed the isoelectric contour. The Tp-e duration was the distance between the two points on the isoelectric line. The Tp-e dispersion was defined as the deviation of the maximum and minimum Tp-e rates. Median heart rate and blood pressure were also recorded for all the groups.
3.3. Statistical Analysis
Descriptive statistics for continuous variables were expressed and tabulated as mean ± standard deviation, and categorical variables were reported as frequencies and percentages (%). Mann-Whitney U tests, and t-tests were used to analyze continuous variables in the two groups. One-way analysis of variance (ANOVA) and post-hoc tests test were utilized to evaluate continuous variables among the groups, and the chi-square test was preferable for qualitative variables in the patient groups. Pearson's correlation test assessed the correlation between the continuous variables. Statistical analysis was performed in the Statistical Package for the Social Sciences (IBM SPSS) v. 21.0 (IBM Corp., Armonk, NY, USA). Statistically significant differences were acceptable when the P-value was less than 0.05. The confidence interval was 95%, and a power analysis was done to determine the number of patients and control participants.
4. Results
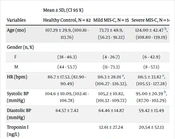
In total, 29 patients (mean age: 104.86 ± 36.86 months, range 12-187 months, 10 females) and 82 healthy controls (38 females, mean age: 107.29 ± 29.90 months, range 25-185 months) were enrolled. There were 15 patients with mild MIS-C and 14 patients with severe MIS-C. In total, 14 patients with severe MIS-C (mean age: 124 ± 42.47 months) had a significantly higher mean age compared to mild MIS-C patients (mean age: 73.73 ± 48.9 months) (P = 0,000). The troponin I level was 12.61 ± 27.24 ng/L and 20,54 ± 52.13 ng/L in patients with mild and severe MIS-C, respectively. While the NT-PRoBNP level was 6642 ± 11326 pg/mL in patients with mild MIS-C, it was 9840 ± 9076 pg/mL in patients with severe MIS-C. The CRP value was elevated in both groups of MIS-C (111.5 ± 65.09 and 163,28 ± 98.77 mg/L in mild MIS-C and severe MIS-C, respectively). The two laboratory parameters, creatinine, and calcium, were significantly different between these two groups, unlike the other parameters. Creatinine was higher in severe MIS-C patients (mild MIS-C 0,43 ± 0.24 mg/dL versus severe MIS-C 0,52 ± 0.18 mg/dL). Calcium was higher in mild MIS-C patients (mild MIS-C 9,02 ± 0.89 mg/dL versus severe MIS-C 8,45 ± 1.22 mg/dL). After analyzing all the ECG parameters, arrhythmia risk seemed prominently higher in severe MIS-C patients than in mild cases (P < 0.05; comparisons are explained at the bottom of Table 1). Mean systolic blood pressure values were found to be significantly low in patients with severe MIS-C compared to the healthy control group (P = 0.019). Diastolic blood pressure values were not prominently different between the patient and control groups (Table 2). Mean heart rates were prominently elevated in all patient groups (P = 0.000), while there was no significant difference in the mean heart rate between the two patient groups. Conventional M-mode echocardiography results of the patient groups are shown in Table 1. We found that the left ventricular systolic function parameters (ejection fraction and fractional shortening) were lower in patients with severe MIS-C compared to the mild MIS-C group (P = 0.044, P = 0.037, respectively). Cardiac troponin I, NT-proBNP, D-dimer, biochemical data, CRP, and sedimentation were also increased in our patients, with MIS-C and NT-proBNP values being significantly higher in patients with severe systolic heart dysfunction (P = 0.001). In the severe MIS-C group, mean serum calcium levels were significantly lower, whereas serum creatinine levels were higher compared to the mild MIS-C group (P = 0.000, P = 0.043, respectively). The clinical, echocardiographic, and laboratory features of both groups, as well as the 95% confidence intervals for all significant quantitative variables, are summarized in Table 2.
| Variables | Mean ± SD, (CI 95%) | ||||
|---|---|---|---|---|---|
| Healthy Controls, N = 82 | Mild MIS-C, N = 15 | Severe MIS-C, N = 14 | MIS-C All, N = 29 | P-Value | |
| PR (ms) | 141.94 ± 18.52, (137.93 - 145.94) | 113.13 ± 28.44 a, (102.95 - 123.30) | 117.2 ± 28.15 a, (107.20 - 127.35) | 115.13 ± 27.87 a, (105.15 - 125.10) | < 0.001 |
| QRS (ms) | 92.44 ± 16.69, (88.82 - 96.05) | 85.06 ± 15.23, (79.64 - 90.47) | 81.2 ± 15.64, (75.60 - 86.79) | 83.21 ± 15.28 a, (77.74 - 88.67) | 0.001 |
| QT (ms) | 341.40 ± 22.97, (336.42 - 346.37) | 352.89 ± 28.59, (342.65 - 363.12) | 373.00 ± 27.04 a, (363.32 - 382.67) | 356.66 ± 29.35, (346.15 - 367.16) | 0.002 |
| QTc (ms) | 415.20 ± 27.07, (409.34 - 421.05) | 434.66 ± 29.06, (424.26 - 445.05) | 439.42 ± 31.87 a, (428.01 - 450.82) | 437.00 ± 29.93 a, (426.29 - 447.71) | < 0.001 |
| Tp-e (ms) | 70.68 ± 8.70, (68.79 - 72.56) | 86.6 ± 20.92 a, (79.11 - 94.08) | 95.64 ± 23.25 a, (87.32 - 103.96) | 90.97 ± 22.16 a, (83.04 - 84.90) | < 0.001 |
| Tp-e dispersion (ms) | 8.78 ± 1.74, (8.40 - 9.15) | 15.46 ± 3.68 a, (14.14 - 16.77) | 19.92 ± 4.05 a,b, (18.47 - 21.36) | 17.62 ± 4.42 a, (16.03 - 19.20) | < 0.001 |
| Tp-e/QTc | 0.20 ± 0.02, (0.19 - 0.20) | 0.25 ± 0.08 a, (0.22 - 0.27) | 0.24 ± 0.07 a, (0.21 - 0.26) | 0.25 ± 0.08 a, (0.22 - 0.27) | 0.004 |
| EF | 71.3 ± 5.43, (69.35 - 73.24) | 59 ± 8.87 b, (55.82 - 61.17) | < 0.05 | ||
| FS | 39.71 ± 3.71, (38.38 - 41.03) | 31.33 ± 6.09 b, (29.15 - 33.50) | < 0.05 | ||
Abbreviations: CI, confidence interval; EF, ejection fraction; FS, fractional shortening; MIS-C, multisystem inflammatory syndrome in children; Tp-e, T-peak to T-end.
a Different from healthy controls (P < 0.05).
b Different from mild MIS-C (P < 0.05).
| Variables | Mean ± SD, (CI 95 %) | ||||
|---|---|---|---|---|---|
| Healthy Control, N = 82 | Mild MIS-C, N = 15 | Severe MIS-C, N = 14 | MIS-C All, N = 29 | P-Value | |
| Age (mo) | 107.29 ± 29.9, (100.81 - 113.76) | 73.73 ± 48.9, (56.23 - 91.22) | 124.00 ± 42.47 b, (108.80 - 139.19) | 98 ± 51.83, (79.45 - 116.54) | < 0.05 |
| Gender, No. (%) | 0.368 | ||||
| F | 38 (46.3) | 4 (26.7) | 6 (42.9) | 10 (34.5) | |
| M | 44 (53.7) | 11 (73.3) | 8 (57.1) | 19 (65.5) | |
| HR (bpm) | 86.7 ± 17.52, (82.90 - 90.49) | 116.3 ± 28.01 a, (106.27 - 126.32), | 116.5 ± 33.82 a, (105.53 - 127.28) | 116.41 ± 30.38 a, (105.53 - 127.28) | 0.000 |
| Systolic BP (mmHg) | 104.6 ± 10.09, (102.41 - 106.78) | 105,2 ± 10.82, (101.32 - 109.72) | 95.00 ± 20.39 b, (87.70 - 102.29) | 100.27 ± 16.69, (94.29 - 106.24) | 0.019 |
| Diastolic BP (mmHg) | 64.57 ± 7.42 | 64.46 ± 14.87 | 59.42 ± 13.49 | 62.03 ± 14.20 | 0.178 |
| Troponin I (ng/L) | 12.61 ± 27.24 | 20,54 ± 52.13 | 0,608 | ||
| NT-Pro BNP (pg/mL) | 6642 ± 11326 | 9840 ± 9076 | 0.411 | ||
| Sedimentation (mm/h) | 53,35 ± 37.35 | 57,06 ± 40.09 | 0.799 | ||
| CRP (mg/L) | 111.5 ± 65.09 | 163,28 ± 98.77 | 0.110 | ||
| PCT (ng/mL) | 5,11 ± 10.97 | 13,90 ± 19.89 | 0.156 | ||
| WBC (microL) | 10712 | 12492 | 0.365 | ||
| Creatinine (mg/dL) | 0.43 ± 0.24, (0.34 - 0.51) | 0.52 ± 0.18 b, (0.45 - 0.58) | 0.043 | ||
| Ddimer (µgFEU/mL) | 2,82 ± 2.46 | 21.18 ± 35.53 | 0.222 | ||
| Calcium(mg/dL) | 9.02 ± 0.89, (8.70 - 9.33) | 8.45 ± 1.22 b, (8.01 - 8.88) | 0.000 | ||
Abbreviations: CI, confidence interval; CRP, C-reactive protein; HR, heart rate; NT-ProBNP, N-terminal pro-brain-natriuretic peptide; MIS-C, multisystem inflammatory syndrome in children; PCT, procalcitonin; Systolic BP, systolic blood pressure; WBC, white blood cell count.
a Different from healthy controls (P < 0.05).
b Different from mild MIS-C (P < 0.05).
4.1. Electrocardiographic Results
The QTc duration was found to be clearly prolonged in the patient group (437 ± 29.93 milliseconds (ms)) than in the control group (415.21 ms ± 25.07; P < 0.01). The Tp-e duration and Tp-e dispersion in both MIS-C groups were noticeably elevated than in the healthy control group (Table 1) (P < 0.01). Moreover, an important difference was found in the Tp-e dispersion between the two patient groups (P = 0.04). This parameter was significantly higher in the severe MIS-C group compared to the patients in the mild MIS-C group. The Tp-e/QTc ratio in both patient groups was significantly higher than the control group (P < 0.001) (Table 1). The PR duration and QRS duration were significantly reduced in the patient group than in the control group (P < 0.01). The QT duration was positively correlated with troponin I in the patient group (P = 0.003). D-dimer values were positively correlated with the length of hospitalization.
During the disease, QTc was remarkably prolonged in 6 patients, among whom it was over 470 MSN in 5 cases and over 500 MSN in just 1 patient. Besides, nonsustained ventricular tachycardia occurred in 2 patients. In all of these patients, ventricular repolarization parameters were notably high. Both patients with VT had severe MIS-C, and their ages were higher (16 to 17 years old). Associated ST-segment changes were detected in 10 patients, and only 1 of them had myocardial infarction related to right coronary artery thrombosis. This patient was treated by stent implantation in angiography. There was no mortality in the patient groups. We started beta-blocker therapy with propranolol in these selected patients with high risks of cardiac arrhythmia after stabilizing their circulatory parameters. These patients showed normal ECG findings after discharge, and the medications were stopped. The ECG parameters of the groups and 95%confidence intervals for all significant quantitative variables are presented in Table 1.
5. Discussion
Multisystem inflammatory syndrome in children is a hyperinflammatory condition with multi-organ engagement after SARS-CoV-2 infection. We conducted a study to evaluate laboratory biomarkers and MIS-C-induced pan-myocarditis using Tp-e distance, Tp-e dispersion, and Tp-e/QTc as an indicator for abnormal repolarization of the heart muscle due to inflammation. This is the first study in which the risk of arrhythmia due to ventricular repolarization changes in MIS-C patients was evaluated by ECG parameters. The QT interval, QTc duration, Tp-e distance, Tp-e dispersion, and Tp-e/QTc ratio in the patient group were significantly higher than in the control group. Moreover, in the severe MIS-C group, the Tp-e dispersion duration was longer than in the mild MIS-C group. The QT duration was correlated with troponin I in the patient group.
Multisystem inflammatory syndrome in children is a complicated disease with fever, elevated inflammatory markers, and multi-organ dysfunction. Cardiovascular problems are quite common in MIS-C and determine the prognosis of the disease. Still, 50% of children with MIS-C could be in circulatory failure with marked hypotension, and 50 - 80% of them must be admitted to the intensive care unit (11, 16). Cardiac involvement occurs in 67 - 80% of children with MIS-C (11, 17-19). The most common cardiac pathologies are coronary artery dilation and aneurysm, myocardial dysfunction, and arrhythmia (11, 17-19). Arrhythmia and conduction abnormalities occur in 28 - 67% of MIS-C patients (6, 20, 21). QT prolongation and tachyarrhythmia have also been noted (6). Regan et al. defined an analysis of ECGs attained after hospital acceptance and followed up with the patients with MIS-C. QTc prolonged in patients with MIS-C during hospitalization. At the same time, they observed minor changes in cardiac repolarization (6). The mean QTc was beyond the normal limits in this study, and only 5 cases had a QTc value over 470 msec. QTc became normal in these patients at discharge. Still, there are just a few studies about ECG parameters predicting prognosis in patients with MIS-C. To the best of our knowledge, it is the first study regarding trans myocardial repolarization parameters in MIS-C.
Based on recent studies, it is known that MIS-C could evoke not just myocardial dysfunction but also cardiac conduction anomalies through direct viral cardiomyocyte destruction, inflammation, and microvascular dysfunction (21, 22). QT interval duration is not always a precise predictor for cardiac lethal arrhythmias (23). T wave morphology assessment could increase predictability. Tp-e might be the best predictor for ventricular repolarization deterioration. This indicator was used when the QTc interval was not increased, or the patient had a long QRS duration. Tp-e interval, Tp-e dispersion, and Tp-e/QTc ratio are new arrhythmia parameters defining transmyocardial heterogeneity (24-26). Some other reports show that the Tp-e interval, Tp-e dispersion, and Tp-e /QTc ratio are ascendant to the QT interval and QT dispersion in predicting ventricular arrhythmias (23, 24, 27, 28). Tp-e is prolonged in the congenital long QT syndrome and forecast Torsades de pointes (24, 25).
We investigated the ventricular repolarization variations in MIS-C after COVID-19 in comparison with control subjects. In our study, QT and QTc measurements, Tp-e interval, Tp-e dispersion, and TpTe/QTc ratio were prominently higher in the patient group than in the control group. These results show that ventricular repolarization had already been affected in children with MIS-C. Besides, it could be detected from even the first ECG. Moreover, in the severe MIS-C group, Tp-e dispersion duration was longer than in the mild MIS-C group. It is known that Tp-e dispersion is a strong predictive parameter detecting ventricular arrhythmias (24-26, 28).
The QTc values were over 470 msn in 5 patients with severe MIS-C in our study. The QTc was over 500 msn in 1 patient, and 2 patients with severe MIS-C had nonsustained ventricular tachycardia. In these patients with an elevated risk for arrhythmia, we started beta-blocker therapy (propranolol), considering blood pressure. It could be stated that ventricular arrhythmia risk could rise, especially in the severe MIS-C group. All these results confirm the strong relationship between ventricular repolarization impairment and severe MIS-C with cardiac complications.
Cardiac disorders in MIS-C patients may be associated with the elevation of both cardiac biomarkers (troponin) and other cardiac function-related proteins (NT-proBNP) by 33.3% and 43.6%, in order (21). We found that the left ventricular function was significantly lower in patients with severe MIS-C compared to the mild MIS-C group. In addition, NT-proBNP levels were significantly higher in patients with seriously affected cardiac functions, which is compatible with the literature. Cardiac troponin I, NT-proBNP, D-dimer, and inflammatory markers were also notably increased in our patients with severe MIS-C. D-dimer values were positively correlated with the length of hospitalization of the patients. Our results demonstrated a close association with the increased inflammatory markers, cardiac enzymes, and severity of MIS-C via cardiac complications. Even if all these markers were higher in the severe MIS-C, there was no statistically significant difference. This could be because of the small sample size of this study. Prospective academic studies with a larger population are needed to confirm arrhythmia risk with these repolarization parameters.
QT duration and troponin I level were positively correlated in our study. The result of this study suggested that the prognosis and severity of the disease could be predicted by the assessment of ventricular repolarization parameters, which were also significantly correlated with the cardiac biomarker levels. It is not clear in whom MIS-C would have a severe trend, as the immune pathophysiology is not exactly explained either. Multisystem inflammatory syndrome in children could be related to a life-threatening cardiac involvement and, thus, an elevated mortality ratio in children.
It could be stated that cardiovascular complications raising the risk of sudden cardiac deterioration appear much more in MIS-C, according to Kawasaki disease. The mechanism for the change in ventricular repolarization could be multifactorial. In some cases, elevated cardiac enzymes show cardiomyocyte injury. These patients have similar clinical signs to those of myocarditis. When the ventricular function is diminished, but cardiac enzymes are normal, alternate pathogenesis could be considered as generalized inflammation. Rare autopsy studies on MIS-C patients have proved inflammation of the endocardium, myocardium, and pericardium, as well as necrosis (22). In fact, unlike Kawasaki disease, MIS-C affects older age groups; moreover, it progresses more severely in this age group. Therefore, a basal ECG should be taken in MIS-C patients to assess the risk and prognosis. Closer ECG monitoring should be considered, especially in severe MIS-C patients.
5.1. Limitations
There are limitations because of the small number of patients in this study. We know that MIS-C is a new and rare disease. The study was carried out at a single center. A prospective study with a large sample should thus be planned. Nevertheless, the ECG data were potentially useful for assessing the risks of the disease.
5.2. Conclusions
Acute cardiovascular involvement is quite common in MIS-C. The related causes and long-term consequences are still being investigated. Our results suggested that MIS-C associated with COVID-19 has a significant effect on the T wave, and these changes may be strongly connected to prognosis. Laboratory biomarkers also correlated with the ECG parameters. A baseline ECG should be taken, and ECG monitoring should be performed in all children diagnosed with MIS-C. Patients with MIS-C must be admitted to intensive unit care due to multi-organ dysfunction. Patients with ventricular repolarization impairment in the ECG should be closely monitored for arrhythmias and sudden death. Effective treatment should be given to these selected patients as well.