1. Background
Secundum atrial septal defect (ASD) is one of the most common congenital heart diseases. Transcatheter device closure of secundum ASD was introduced in 1974 by King and Milk (1). Device closure has become the preferred method for most defects due to its advantages, including a reduced burden of trauma for the patient, avoidance of cardiopulmonary bypass, and a more favorable cosmetic outcome compared to surgery (2, 3). According to large cohorts, the risk of serious complications is very low (1.8 %) (3), and mortality is extremely rare (0.093%) in this method (4).
The co-existence of a metabolic or genetic disease can alter the situation, introducing risks ranging from mild to severe complications and even death. Understanding these risks is crucial when planning ASD closure in patients with comorbidities.
2. Objectives
To the best of our knowledge, this particular subject has not been addressed in the medical literature before. Hence, we are sharing our experience with ASD closure in these patients over a period spanning more than 16 years in a single center.
3. Methods
In this retrospective study, the medical records of 188 ASD device closure attempts performed at our hospital between September 2006 and December 2022 were searched for patients with a co-existing metabolic or genetic disease. A total of 11 patients were identified and included in this case series. Demographic characteristics, details of the comorbid disease, procedure-related complications, procedure outcome, device usage, and follow-up data were collected. Any change in the procedure (anesthesia, ASD closure procedure, post-procedure care) was documented. Furthermore, consultations with other specialists were sought based on the specific comorbidities. The main objective was to record any variation in various aspects of the procedure in these patients.
The study protocol was approved by the Ethics Committee of Tehran University of Medical Sciences. Informed consent was obtained from the parents or guardians. The frequencies of major complications were compared between the study group (complex ASD: ASD with genetic or metabolic disease) and the control group (simple ASD: ASD without genetic or metabolic disease).
Atrial septal defect closure was performed in accordance with previously established protocols (2, 5-7). Initially, balloon sizing was routinely employed (2, 5). Over time, for the majority of patients, balloon sizing was gradually phased out, with the size of the defect determined through transesophageal echocardiography (TEE) or three-dimensional transthoracic echocardiography (6). Clopidogrel was administered for a duration of 3 months, and aspirin was continued for one year following the procedure. Scheduled follow-up visits were arranged at 1 week, 1 month, 3 months, and 6 months post-procedure. Subsequently, follow-up assessments were conducted every 6 months until reaching the 3-year mark after the procedure, at which point they transitioned to an annual schedule. During these visits, echocardiography and electrocardiograms were routinely performed. Data are expressed as median and range whenever applicable. The chi-square test was used to compare complication rates. P values less than 0.05 were considered statistically significant.
4. Results
4.1. Patient Characteristics
The patients' characteristics are summarized in Table 1. The median age of the patients was 4.5 years (range: 1.66 - 14 years), and their median weight was 15 kg (range: 4 - 94 kg). Among patients with genetic syndromes, there were two with Alagille syndrome, one with Seckel syndrome, and one with Down syndrome. One patient had insulin-dependent diabetes mellitus, and one patient suffered from obesity and insulin resistance. The remaining patients presented with various conditions, including mitochondriopathy, rickets, Crigler Najjar syndrome type II, combined oxidative phosphorylation deficiency-35, and cystic fibrosis.
| Patient | Age (y) | Weight (Kl) | Comorbid Disease | Complications | Device Type | Device Size (s) | Success | WWR | F/U |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 26 | IDDM | Cardiac thrombi, device embolization | ASD-R | 16 & 18 | - | - | 5 |
| 2 | 4 | 9 | Mitochondrial disease | - | Starway | 14 | + | 1.56 | 11 |
| 3 | 5 | 15 | Rickets | - | Starway | 14 | + | 0.93 | 6 |
| 4 | 4 | 4 | Seckel syndrome | - | Figulla | 9 | + | 2.25 | 7.5 |
| 5 | 3 | 17 | Alagille syndrome | - | Figulla | 16.5 | + | 0.97 | 2.5 |
| 6 | 3 | 11 | Crigler Najjar syndrome type II | - | Amplatzer | 17 | + | 1.55 | 1.5 |
| 7 | 14 | 94 | Metabolic syndrome | Ischemic stroke (right MCA) | Figulla | 33 | + | 0.35 | 1.5 |
| 8 | 4.5 | 11.5 | Combined oxidative phosphorylation deficiency-35 | - | Figulla | 12 | + | 1.04 | 0 |
| 9 | 8 | 20 | Cystic Fibrosis | - | Figulla | 24 | + | 1.2 | 4 |
| 10 | 5 | 16 | Down syndrome | - | Figulla | 9 | + | 0.56 | 4 |
| 11 | 1.66 | 7.5 | Alagille syndrome | Bleeding | Amplatzer | 11 | + | 1.46 | 0 |
Abbreviations: WWR, Device Waist to Body Weight Ratio; F/U, Follow-up; IDDM, Insulin-Dependent Diabetes Mellitus; MCA, Middle Cerebral Artery; ASD-R (pfm AG, Germany); Starway (Starway Cardi-O-Fix ASD Occlude, Starway Medical Technology, China); Figulla ((Figulla flex ASD occluder, Occlutech, Sweden); Amplatzer (Amplatzer Septal Occluder, Abbott Laboratories, USA).
4.2. Procedural Details
Figulla Flex ASD occluders (Occlutech, Sweden) were used in 6 patients, while Starway Cardi-O-Fix ASD occluder (Starway Medical Technology, China) and Amplatzer Septal Occluder (Abbott Laboratories, USA) were each used in 2 patients. An ASD-R device (pfm AG, Germany) was implanted in one patient. The median size of the implanted devices was 14 mm (range: 9 - 33 mm). The procedure was successful in 10 patients (91%).
4.3. Complications
One patient (Patient 1) required surgical intervention due to device embolization, which was accompanied by the presence of a cardiac thrombus during the procedure. No other major complications occurred shortly thereafter. Another patient experienced a stroke 9 months post-procedure. In Table 2, the rate of these complications is compared with those associated with ASD device closure in otherwise normal patients.
Abbreviation: ASD, atrial septal defect.
a Statistically significant
4.4. Follow-up
The median follow-up duration was 4 years (range: 0 - 11.5 years). One of the patients experienced an ischemic stroke in the territory of the right middle cerebral artery during the follow-up period (Figure 1). One patient passed away 19 days after the procedure due to multi-organ failure affecting the hepatic, renal, and cerebral systems. The death was unrelated to the ASD closure. Three major complications (device embolization, cardiac thrombus, and ischemic stroke) occurred in the study group, while none of these complications were observed in the control group (Table 2, P<0.0001). Detailed data for each patient is provided in the following.
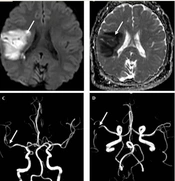
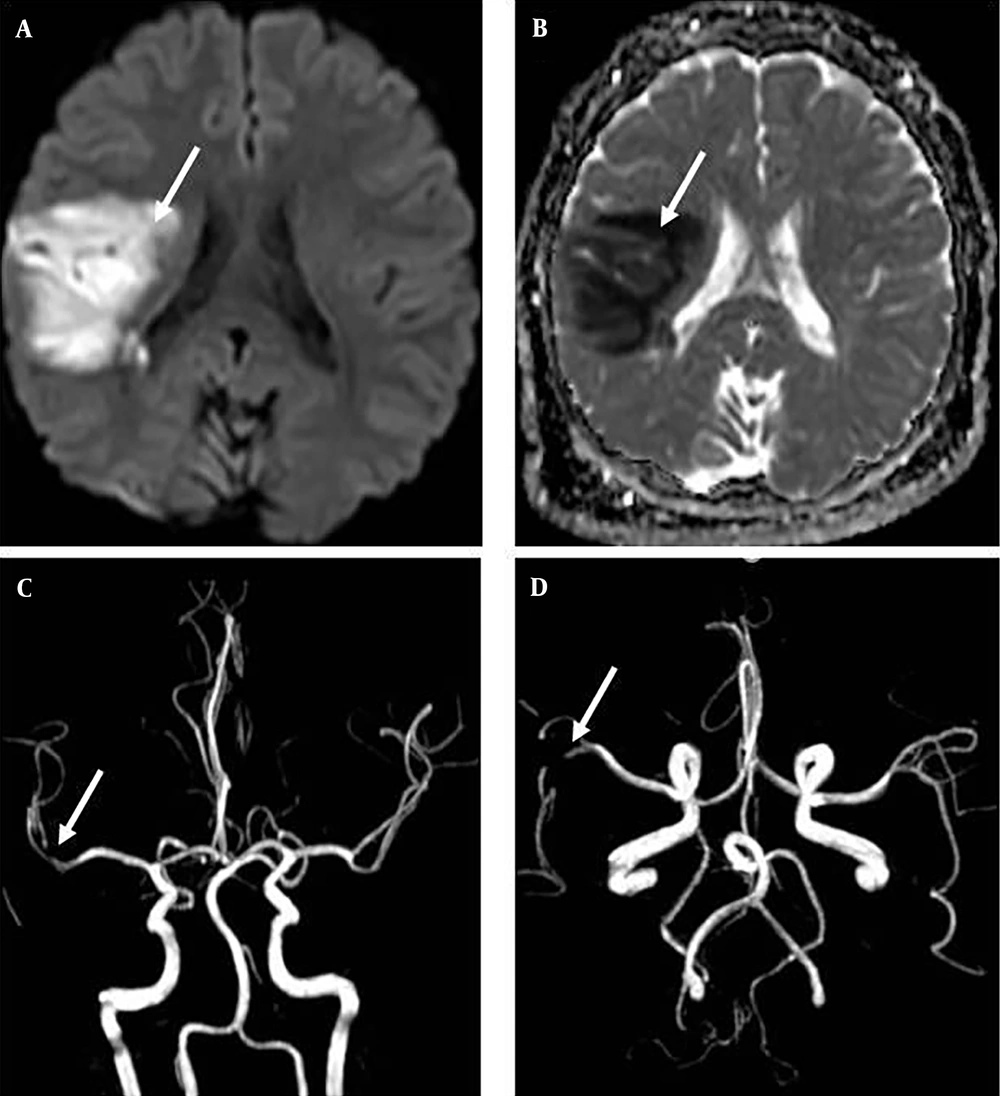
Brain magnetic resonance imaging of patient 7 (A, B): A, axial diffusion-weighted imaging, B, axial apparent diffusion coefficient; brain magnetic resonance angiography of patient 7 (C, D) revealing large areas of diffusion restriction around the right sylvian fissure, indicating an ischemic event in the territory of the right middle cerebral artery (A, B, C, D: White Arrow).
4.5. Case Reports
4.5.1. Patient 1
A 12-year-old girl with large ASD and insulin-dependent diabetes mellitus was referred to our hospital for ASD device closure. Despite insulin therapy, her diabetic control was poor, indicated by elevated blood glucose and HbA1c levels (fasting blood glucose: 243 mg/dl, HbA1c: 8%). As part of her pre-procedure care, she was admitted to the hospital, and her blood glucose levels were successfully normalized. Echocardiography revealed an approximately 12-13 mm defect with adequate rims.
In the catheterization laboratory, she received 100 units/kg of heparin. The ASD size was measured using a 25 mm Occlutech sizing balloon (Occlutech, Sweden) and the waist method, which determined the defect size to be 15.5 mm. An attempt was made to implant a 16 mm ASD-R device (pfm AG, Germany), but the device passed through the defect too easily. Per our protocol for this situation, a decision was made to utilize a device two sizes larger, resulting in the selection of a 20 mm ASD-R. During this stage, the presence of a large mass on the right atrium, which seemed to originate from the septum between the defect and the tricuspid valve, was noted on echocardiography. It was suspected that the mass was a thrombus. Given a low activated clotting time (ACT) of 170 seconds, additional heparin was administered, raising the ACT to over 300 seconds. Since a larger sheath was required to accommodate the 20 mm ASD-R device (from 10 to 12 Fr) and considering the potential risk of thrombus embolization during a lengthy sheath exchange, a decision was made to attempt an 18 mm ASD-R occluder. The device was successfully implanted and positioned appropriately.
Late in the afternoon, the patient developed frequent premature ventricular beats in the intensive care unit. Subsequent echocardiography revealed that the device had migrated to the right ventricle. The patient underwent surgery the following day. During the procedure, damage to the pulmonary and tricuspid valves was identified and subsequently repaired. The device and thrombus were successfully removed, and the defect was closed. The surgeon confirmed that the thrombus had originated from the interatrial septum. The postoperative course proceeded favorably without any neurologic or cardiac complications. This incident represented the only ASD device embolization occurrence during the study period.
4.5.2. Patient 2
The patient's metabolic condition was diagnosed as mitochondrial disease, characterized by symptoms including growth retardation, developmental delay, microcephaly, brain atrophy, and a history of cataract surgery. Her laboratory data consistently indicated elevated lactate levels and high anion gap acidosis. The procedure was performed when she was 4 years old. Although both her weight (9 kg) and the device waist-to-body weight ratio (WWR, 1.56) were slightly outside the recommended safe range, the procedure was carried out without any complications. Over the year following the procedure, she experienced a weight gain of 4 kilograms.
4.5.3. Patient 3
A patient with vitamin D-resistant rickets receiving vitamin D3, calcium supplements, and dihydrotachysterol (AT 10) was referred for ASD device closure. The defect was of moderate size, and pulmonary artery pressure was within the normal range. The procedure was successfully completed without any adverse events.
4.5.4. Patient 4
A patient with severe growth retardation (birth weight: 700 gr, weight at 4 years of age: 4 kg), along with microcephaly, developmental delay, facial abnormalities, and strong clinical suspicion of Seckel syndrome, was followed up since infancy. Seckel syndrome, initially described in 1960 by Seckel, is a genetic syndrome characterized by severe growth retardation, moderate to severe mental retardation, and distinctive craniofacial features, including a beaky and protruding nose, micrognathia, large eyes, malformed ears, a narrow face, and microcephaly. Diagnosis of Seckel syndrome is primarily clinical in nature (8). Congenital cardiac diseases are not a feature of the syndrome.
The patient was continuously monitored, and ultimately, at the age of 4 years, the decision was made to close the atrial septal defect (ASD). This choice was influenced by the expectation that significant weight gain in the following years was unlikely. At the time of the procedure, the patient's weight (4 kg) and waist-to-weight ratio (2.25) were considerably outside the recommended safe ranges. Nonetheless, the closure of the defect was carried out successfully, and the patient experienced no issues over the subsequent 7 years post-procedure. His weight increased to 6.7 kg during this period.
4.5.5. Patient 5
In a patient diagnosed with Alagille syndrome, characterized by cholestatic jaundice and bile duct paucity, the ASD was successfully closed using a 16.5 mm device. The procedure transpired without any adverse events. Notably, the pulmonary artery branches displayed normal size, and the ASD represented the patient's sole cardiac anomaly.
4.5.6. Patient 6
This patient had been experiencing jaundice since the neonatal period, along with hypothyroidism and growth retardation. A clinical diagnosis was established, indicating Crigler-Najjar type II syndrome, which is a less severe form of the syndrome. Importantly, the pulmonary artery pressure remained within the normal range. To close the atrial septal defect (ASD), a device with a slightly higher waist-to-weight ratio (WWR) than usual (1.55) was employed, and the ASD was successfully occluded without any complications.
4.5.7. Patient 7
The patient, a 14-year-old boy, presented with severe obesity (body mass index: 36, Z score: 4.2), hypertriglyceridemia, clinical symptoms suggestive of insulin resistance, and an impaired glucose tolerance test. Following the successful closure of the atrial septal defect (ASD), he was discharged with the standard recommendation of taking clopidogrel for 3 months and aspirin for one year. However, he intentionally discontinued the use of aspirin after 9 months. Two weeks later, he experienced left hemiparesis. Magnetic resonance imaging revealed extensive areas of diffusion restriction surrounding the right Sylvian fissure, indicative of an ischemic event within the territory of the right middle cerebral artery (Figure 1). Subsequently, he exhibited gradual improvement. During the follow-up period, his weight decreased as he initiated a weight-reduction regimen.
4.5.8. Patient 8
In a patient presenting with severe growth retardation, global developmental delay, a history of seizures, and a confirmed diagnosis of combined oxidative phosphorylation deficiency-35, the ASD was successfully closed. Mitochondria are known to play a critical role in intracellular calcium metabolism and may also have a subsidiary role in the progression of malignant hyperthermia (9). To mitigate the risk of malignant hyperthermia, a series of precautions were taken during the administration of anesthesia. Prior to using the anesthesia machine, the anesthesia circuit was flushed with oxygen for a period of 2 hours. The carbon dioxide absorber was replaced before inducing anesthesia. Induction was initiated with midazolam and fentanyl, while propofol infusion and cisatracurium were used for maintenance. Arterial blood gas (ABG) levels were monitored twice during the procedure, immediately after the start of anesthesia and before extubation. All ABG parameters remained within the normal range, with no signs of metabolic disturbances. The patient was successfully extubated, awakened, and transferred to the intensive care unit with stable vital signs. It is noteworthy that all general anesthetics studied thus far have been shown to depress mitochondrial function (10). To minimize the risk of malignant hyperthermia, inhaled gases were avoided. Additionally, due to the patient's history of recent seizures during hospitalization, ketamine infusion was not used. Ultimately, propofol infusion was selected for the maintenance of anesthesia. Importantly, it should be noted that both the procedure and anesthesia were of relatively short duration.
4.5.9. Patient 9
Cystic fibrosis was diagnosed in the patient due to the presence of growth retardation, steatorrhea, and salty sweat. The diagnosis was confirmed through a sweat test and genetic analysis. The ASD was identified during an echocardiographic assessment of pulmonary artery pressure and right ventricle function. The pulmonary artery pressure was within the normal range, and the ASD was successfully occluded.
4.5.10. Patient 10
The karyotype confirmed Down syndrome. The patient presented with an ASD of borderline size (maximum size: 6.5 mm, shunt ratio: 1.7) and normal pulmonary artery pressure. Consequently, a decision was made to proceed with the closure of the defect. The procedure was performed without any complications.
4.5.11. Patient 11
A 20-month-old patient with Alagille syndrome (confirmed NOTCH2 mutation), hepatic failure (cholestatic jaundice, coagulation abnormalities (International Normalized Ratio: 2.6), thrombocytopenia), a large secundum ASD measuring 8 mm with a deficient aortic rim, and characteristic abnormal facial features was referred for ASD device closure. This patient was a candidate for hepatic transplantation; however, given the preference for a higher weight (at least 10 kg) and the repair of the cardiac condition before transplantation, ASD device closure was attempted. Assessment of the pulmonary artery and its main branches revealed normal sizes. The right ventricle exhibited dilation with normal systolic pressure. To prepare the patient for the procedure, fresh frozen plasma (FFP) and platelet transfusions were administered. FFP infusion was repeated during the procedure and later due to bleeding at the femoral puncture site a few hours after hemostasis, as well as ongoing pharyngeal hemorrhage. The ASD was successfully occluded using an 11 mm Amplatzer Septal Occluder (Abbott Laboratories, USA), guided by transthoracic echocardiography. No anticoagulation therapy was initiated for the patient. Four days following the procedure, the patient was discharged without any bleeding complications.
Seventeen days later, she was admitted to the hospital due to edema, unconsciousness, severe abdominal distention, oliguria, and respiratory distress. Upon evaluation, there were concerns of hepatic encephalopathy with hepatorenal syndrome or intracranial hemorrhage. Echocardiography revealed that the device was appropriately positioned without any signs of thrombus, valvar regurgitation, or cardiac dysfunction. The cardiac rhythm remained normal. Despite all the medical interventions and efforts, her condition deteriorated, and she passed away from her illness after 2 days.
5. Discussion
While this cohort represents a small percentage of ASD closures during the study period (6%), it encompasses some of the most challenging cases. In our experience, the most serious adverse events in all our patients undergoing ASD device closure occurred in those with concurrent conditions falling within the spectrum of diabetes, insulin resistance, obesity, and metabolic syndrome. It is important to note that patients with metabolic or genetic diseases are not homogeneous. Nevertheless, many of them share common issues that warrant consideration before, during, or after ASD device closure. Some of the challenges we encountered within our cohort are discussed below.
5.1. Patient Size
A large number of these patients are evaluated at a young age to assess potential cardiac involvement, leading to the early diagnosis of their atrial septal defects (ASD). Many of these patients also experience growth retardation, a condition for which both the metabolic/genetic disease and ASD may share responsibility. It is important to note that while metabolic or genetic diseases are typically challenging to cure, ASD can often be readily treated. In certain cases, such as Seckel syndrome, achieving a desirable weight can be exceptionally challenging.
Atrial septal defect device closure is typically recommended for patients with a weight exceeding 10 kg (11, 12). It is important to consider the potential risks, including cardiac arrhythmias, especially complete heart block, and erosion, associated with ASD closure in small children. However, there have been reports of successful ASD closure in patients of smaller stature (11, 12). In two patients (2 and 4), a decision was made to postpone the procedure until they reached the age of 4 years, even though their weight remained below 10 kg. Both procedures were conducted successfully and without complications.
In addition, a WWR exceeding 1.5 is generally regarded as a high-risk procedure (13). There are reports of safe ASD closure with devices larger than 1.5 times the patient’s weight (13). We had three patients with a WWR exceeding 1.5 (patients 2, 4, and 6; two of whom also weighed less than 10 kg). The procedure was successful in all of these cases. It can be assumed that ASD device closure in these small patients does not carry a much higher risk if it is postponed to at least 4 years of age.
5.2. Hypercoagulation
Thrombus formation on the device is a major complication of the procedure. It may be fatal or require surgical device removal (14). Hypercoagulation with any cause can be seen in metabolic diseases, and it may predispose the patient to thrombus formation. It is a frequent complication of diabetes mellitus and insulin resistance (15). There were two patients with these abnormalities, and both experienced significant thromboembolic events. It may be advisable to pursue aggressive control of diabetes prior to the procedure, employ higher levels of anticoagulation with careful monitoring of ACT during the procedure, and extend the duration of antiplatelet therapy.
5.3. Bleeding Tendency
Metabolic or genetic diseases can affect coagulation. Coagulation abnormalities, as seen in patient 11, may severely complicate the procedure. Careful attention and sufficient transfusions are required. Striking a balance is crucial, as hypercoagulation can present risks as well.
5.4. Anesthesia
Metabolic diseases encompass a group of congenital conditions that can present significant challenges in the context of anesthesia and surgery (16). These diseases are characterized by their heterogeneity, with unique anesthesia considerations applicable to each. Anesthesiologists may encounter issues such as hepatic disease, difficult intubation, the risk of malignant hypertension, and renal disease. It is crucial to exercise diligent control over water and electrolyte balance, as well as blood sugar levels (17, 18). Continuous ABG monitoring can be employed to assess and manage electrolyte disturbances and metabolic abnormalities.
Patients with mitochondrial disease are prone to elevated lactate levels and subsequent acidemia when subjected to prolonged fasting. As a result, it is advisable to refrain from prolonged fasting in these patients (10). In the context of mitochondrial disorders, it is important to note that nearly all general anesthetics, whether volatile or parenteral, can depress mitochondrial function (19). Nonetheless, certain studies have advocated for bolus doses of propofol in these cases. It is worth mentioning that the use of propofol infusion is not recommended and should only be employed for a brief duration (20).
Atlanto-occipital instability and macroglossia are specific problems of patients with Down syndrome that may increase the risk or difficulty of anesthesia. Patients with severe pulmonary diseases, such as those with cystic fibrosis, may encounter challenges related to general anesthesia and pulmonary care. In some instances, individuals with these conditions may also experience pulmonary hypertension, which can complicate anesthesia and influence the decision-making process regarding ASD closure.
5.5. Echocardiographic Guiding
Echocardiography is typically employed to guide the interventionist during ASD device closure. Transesophageal echocardiography is the most commonly utilized method. However, its use may be limited due to factors such as bleeding tendencies, esophageal or laryngeal diseases, and small patient size in individuals with metabolic or genetic diseases (21). While transthoracic echocardiography is less commonly utilized, it can be safely used in these situations (2). For patient 11, the presence of severe coagulopathy discouraged the use of transesophageal echocardiography.
5.6. Heart Failure
Cardiac failure following ASD device closure is a clinical concern more commonly observed in adults and rarely in children. However, in some cases, it can occur in children with compensated cardiac failure and metabolic disease after ASD device closure (22).
5.7. Renal Function
Patients with genetic or metabolic diseases may be at risk of renal function impairment, which can potentially be exacerbated by the use of contrast media. Fortunately, in contemporary practice, angiography has been largely replaced by echocardiography, and the use of contrast media is now limited during ASD device closure.
5.8. Atrial Septal Defect Closure in Genetic Diseases
Atrial septal defect is not a common feature of Seckel syndrome (8). To the best of our knowledge, our patient was the first case with this syndrome in whom ASD device closure was successfully achieved. Atrial septal defect has been reported in patients with combined oxidative phosphorylation deficiency-35 (23, 24).
Atrial septal defect is one of the cardiac diseases seen more frequently in patients with Down syndrome. The prognosis of ASD closure is excellent and comparable to genetically normal patients if pulmonary vascular disease is not present at the time of closure (25). Congenital cardiac malformations are identified in 94% of patients with Alagille syndrome (26). Atrial septal defect is not one of the most common cardiac malformations associated with the condition, but the link between the two is well established. Successful ASD device closure in Alagille syndrome has already been reported in the medical literature (27).
5.9. Limitations
The major limitations of this study stem from its retrospective nature and the absence of certain data. Additionally, the follow-up duration was relatively short for some patients. The cohort was heterogeneous, as our target group encompassed all patients with genetic or metabolic diseases rather than focusing on a specific disease. Nevertheless, we presented data on various diseases, and they can serve as individual case reports.
5.10. Conclusions
Atrial septal defect device closure is a viable option for patients with metabolic and genetic diseases, provided that special attention is given to their non-cardiac conditions and the potential procedural risks under specific circumstances.