1. Background
Branch pulmonary artery stenosis may occur either isolated or in association with other cardiovascular malformations. In 2% to 3% of the patients, this condition is associated with congenital heart diseases. In addition, it may develop secondary to surgical procedures, for example, after the surgical repair of tetralogy of Fallot (1, 2). Branch pulmonary artery stenosis may accompany some sporadic or familial chromosomal abnormalities such as Williams syndrome, Allagile syndrome, and Noonan syndrome, which may have an impact on the survival and quality of life of these patients (3). This condition can increase right ventricular afterload and cause right heart failure, leading to the redistribution of pulmonary blood flow, ventilation-perfusion mismatch, and decreased exercise capacity (3, 4).
As pulmonary artery origin become significantly narrow, an overall reduction in pulmonary blood flow or a disproportionate distribution of blood into the lungs could be expected. Indications for intervention include right ventricular peak systolic pressure equal to or greater than 50% of the aortic systolic pressure or predicted ipsilateral lung perfusion of less than 35%, evidenced by pulmonary artery imaging (5).
Balloon angioplasty (BA) and stent implantation (SI) are two catheter-based interventional methods that can increase vessel diameter and decrease the trans-stenotic pressure gradient, which in turn could improve ventilatory efficiency and stroke volume in these patients (4, 6-9).
2. Objectives
Few studies have compared the therapeutic outcomes of SI and BA in the treatment of patients with branch pulmonary artery stenosis type 3 (2, 9, 10), so in this study, our main objective was to compare the midterm results of SI and BA in children and adolescents with isolated branch pulmonary artery stenosis.
3. Methods
This project was designed as a cross-sectional study conducted on patients with branch pulmonary artery stenosis referred to the Namazi tertiary center affiliated with Shiraz University of Medical Sciences, Shiraz, Iran, for whom the transcatheter intervention was performed consecutively between January 2010 and January 2019. The procedures were performed after obtaining the informed consent of all participants’ parents and the approval of the Ethics Committee of Shiraz University of Medical Sciences. This study was performed according to the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The ethics committee code number was IR.SUMS.MED.REC.1398.365.
Patients’ demographic data, including age, gender, and body weight, were recorded.
Standard procedures for balloon angioplasty (11) and percutaneous stent implantation (12) were applied. The following items were considered inclusion criteria for conducting the procedures: (1) Elevated right ventricular systolic pressure higher than 50% of aortic systolic pressure; (2) relevant stenosis (i.e., the branch pulmonary artery diameter of ≤ 50% of the adjacent vessel, hypoperfusion of the involved lung, and differential pulmonary blood flow according to computerized tomography scan); (3) hypertension of the unobstructed pulmonary artery; and (4) exercise intolerance. Criteria for successful interventions included the following: (1) A decrease of more than 20% in the right ventricular systolic pressure to aortic pressure ratio; and (2) a 50% increase in the diameter of the stenosis. Children with post-operative branch pulmonary artery stenosis and William’s syndrome were excluded from the study (2). The possible diagnosis of William’s syndrome was based on the patient’s genetic testing or the simultaneous presence of supravalvar aortic stenosis.
The procedures were performed under general anesthesia. Standard hemodynamic parameters were measured, and selective angiographies were undertaken.
Since the stent placed in childhood usually cannot be re-dilated to the required diameter in adulthood, we used the BA method in young children and the SI method in older children. In recent years, redilatable stents have been developed, and we have recently started to use them for our patients.
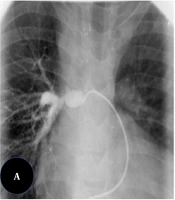
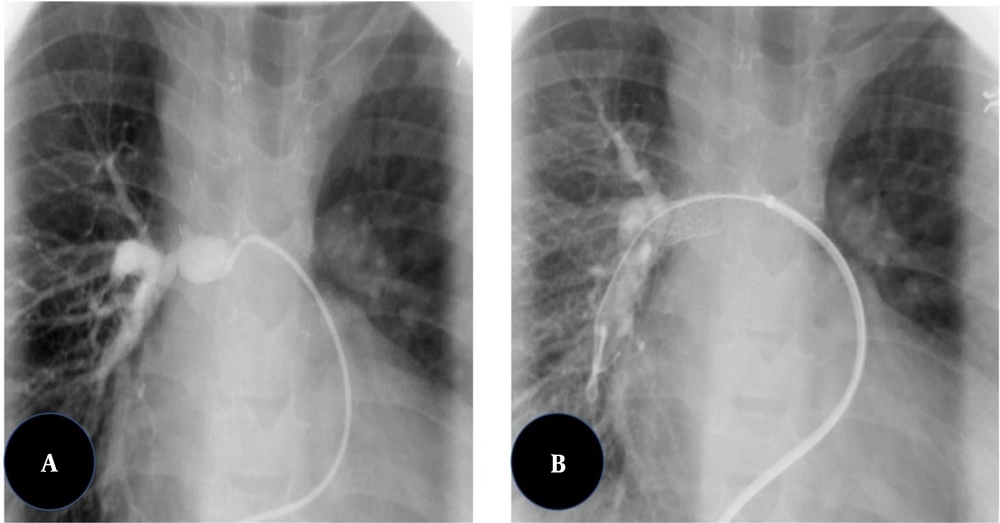
The balloon inflation time was 10 - 60 s, depending on the response of the waist and the degree of interference with cardiac output. Balloons were inflated 2 or 3 times. The diameter of the balloon was chosen to be three or four times larger than the diameter of the narrowed part of the vessel or 1.5 times greater than the diameter of the surrounding pre- or post-stenotic vascular segments. Different brands of balloon catheters were used, but all of them were medium- or high-pressure balloons. The vascular stenting procedure was performed by guiding an anti-kink delivery system over a super stiff exchange wire previously anchored across the stenosis. The stents were mounted on medium- to high-pressure angioplasty balloons with diameters not exceeding the diameter of the vessel adjacent to the stenosis. The delivery system was advanced through the stenosis, and the balloon-stent assembly was advanced within the sheath to the level of the stenosis. Then, the sheath was withdrawn, exposing the balloon-stent assembly (Figure 1).
After stent deployment, subsequent balloon dilations were considered to optimize the outcome if required. Palmaz Genesis peripheral stents (Johnson and Johnson, Warren, NJ, USA), CP stents (NuMED), and ev3 IntraStent LD Max were used most frequently. Post-dilation angiographies and hemodynamic measurements were performed after the interventions (2).
Peak pressures of the right ventricle and the main pulmonary artery on both sides of the stenosis before and after procedures were measured to determine the success rate.
M-mode, two-dimensional, Doppler, and Color-Doppler transthoracic echocardiography procedures were performed before, as well as immediately and every 6 months after the interventions using a 2 - 4 Hz HS70 probe made by Samsung Co. (South Korea) (13, 14). The procedures were performed by pediatric interventional cardiologists.
Patients were divided into 2 groups (balloon angioplasty and stent implantation), and the data of patients who had at least a 2-year follow-up were compared between the 2 groups.
3.1. Data Analysis
For statistical analysis, all variables were initially tested for normality using the Kolmogorov-Smirnov test, all of which showed normal distribution. Qualitative variables were presented as frequencies and percentages, and quantitative variables as mean and standard deviation (mean ± SD), or median, minimum, and maximum. The data were compared using the Mann-Whitney U test. A P value less than 0.05 was regarded as the statistical significance level.
4. Results
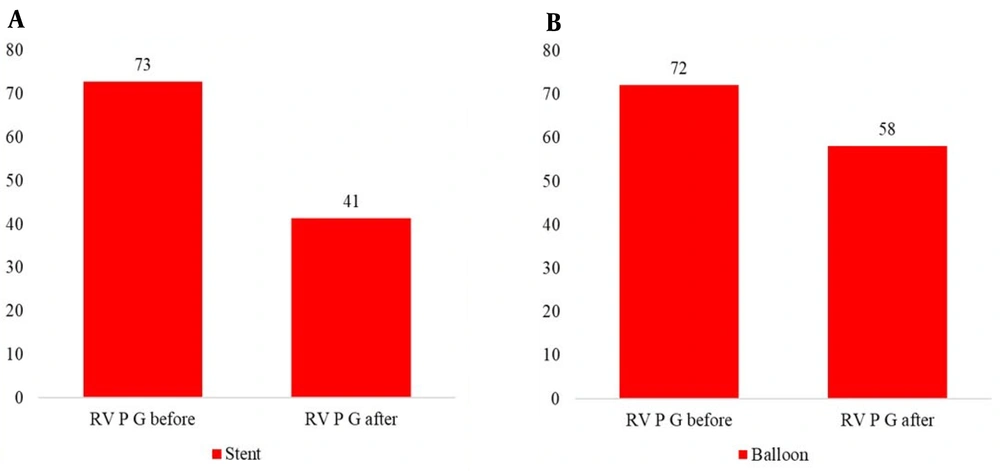
A total of 40 patients (21 males and 19 females) were included in this study. The study population had a median age of 2.1 years (range: 0.1 - 16 years) and a median weight of 10 kg (range: 3 - 42 kg) at the time of the onset of the interventions (Table 1). The peak right ventricular pressure gradient decreased from 75 to 41 mm Hg in the SI group and from 72 to 48 mm Hg in the BA group.
Out of 25 patients undergoing BA, 9 weighed ≤ 10 kg, while out of 15 patients in the SI group, one weighed ≤ 10 kg.
The brands of the balloons used included Passeo (n = 16), Balt (n = 4), Z-MED (NuMED) (n = 2), Admiral Xtreme (n = 2), and EverCross (n = 1). The brands of the stents used included Genesis (cordis) (n = 5), Cook Formula 535 expandable (n = 5), IntraStent Max LD (n = 4), and Valeo biliary stent (n = 1).
The demographic and clinical characteristics of the study participants are shown in Table 1.
| Characteristics | No. (%) | Median | Minimum | Maximum |
|---|---|---|---|---|
| Gender | - | - | - | |
| Male | 21 (52.5) | |||
| Female | 19 (47.5) | - | - | - |
| Age (y) | - | 2.1 | 0.1 | 16 |
| Weight (kg) | - | 10 | 3 | 42 |
| BA | 25 (62.5) | - | - | - |
| SI | 15 (37.5) | - | - | - |
Abbreviations: BA, balloon angioplasty; SI, stent insertion.
Three patients (12%) in the BA group with weights ≤10 kg required re-intervention within 3 months, 1 year, and 2 years after the first procedure, for whom BA was performed again, and they were followed up for 2 years after the second procedure. The location of stenosis in these cases was in a discrete point more often than that observed in other patients. No individual required stent redilation in the SI group.
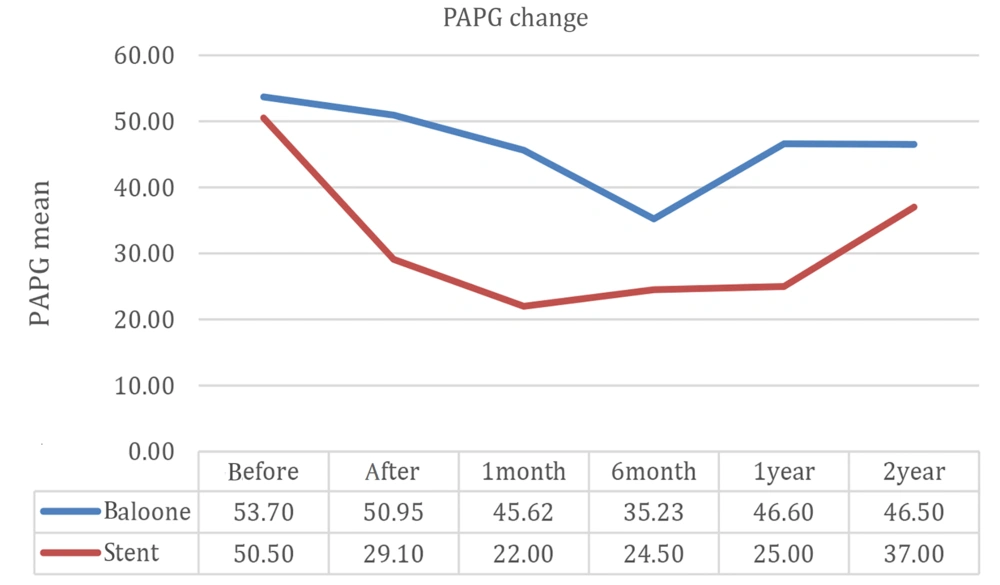
Trans-stenotic pulmonary artery pressure gradient significantly reduced immediately after the procedure in both groups (P = 0.014). This gradient showed a significant difference between the BA and SI groups after one month of the procedures and during the follow-ups (P = 0.57) (Table 2).
| Characteristics | Before the Procedures | After the Procedures | 1 Month | 6 Months | 1 Year | 2 Years |
|---|---|---|---|---|---|---|
| Trans-stenotic pulmonary artery pressure gradient (mmHg ± SD) | 52.73 ± 22.654 | 43.67 ± 21.445 | 39.06 ± 19.456 | 32.71 ± 14.378 | 41.62 ± 18.113 | 45.14 ± 26.086 |
| P- value | 0.014 | 0.570 | 0.432 | 0.655 | 0.715 |
There was a significant decline in the peak right ventricular pressure after both procedures (P = 0.000), but no statistically significant difference was observed between the BA and SI groups (P = 0.18) (Table 3, Figure 2).
| Characteristics | Before the Procedures | After the Procedures | P-Value |
|---|---|---|---|
| Right ventricular pressure (mmHg) | 72.39 ± 26.206 | 54.19 ± 17.477 | 0.0001 |
Figure 3 compares 2-year follow-up outcomes between the BA and SI groups, showing a lower rate of restenosis in the SI group during 1-year follow-up, after which the respective curves in the 2 groups converged.
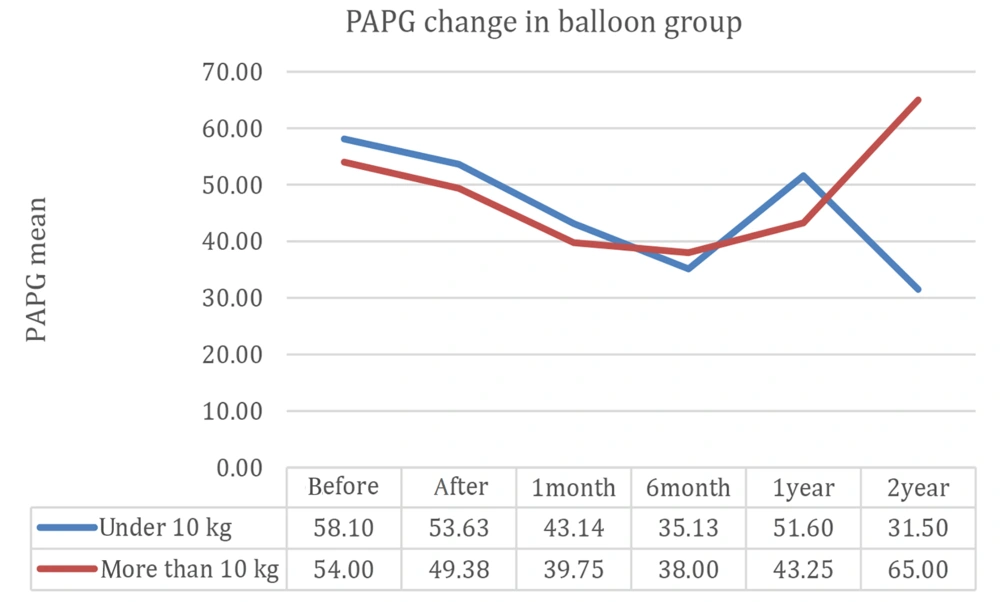
Figure 4 compares 2-year follow-up results between patients weighing less than 10 kg and higher than 10 kg in the BA group, indicating that patients with weights below 10 kg had less stenosis, evidenced by echocardiography during the follow-up, compared to those weighing higher than 10 kg (P = 0.049).
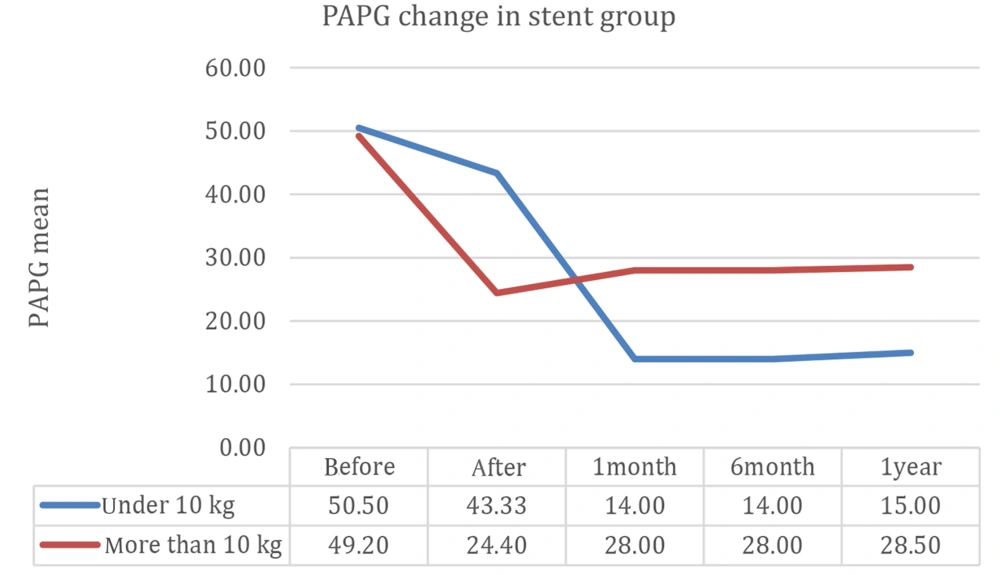
Echocardiographic data after one year of follow-up were compared between patients weighing less than or higher than 10 kg at the time of procedure conduction in the SI group (Figure 5), showing that the 2 curves representing restenosis moved in parallel with each other in these patients.
According to Figure 4, the branch pulmonary artery pressure gradient decreased immediately after BA and continued to decrease for 6 months afterward. After this time, however, the decline continued in patients weighing less than 10 kg, while after 6 months, stenosis gradually increased in patients weighing above 10 kg.
According to Figure 5, the branch pulmonary artery pressure gradient decreased immediately after SI and then remained stable for one year.
There was no mortality during the procedures and within 2 years of follow-up. There was no vascular disruption or dissection in either group. Although these procedures may be associated with adverse events similar to other cardiac catheterization techniques, only one patient showed a complication during SI, for whom the stent was moved forward and then successfully retrieved by snaring, and another stent was implanted instead.
5. Discussion
Severe stenosis of branched pulmonary arteries can cause various complications, including an increase in right ventricular pressure, leading to higher myocardial oxygen demands and, subsequently, myocardial hypertrophy, heart failure, and cardiac dysfunction. So, it is essential to control right ventricular pressure in patients with pulmonary artery stenosis.
Pulmonary artery stenosis is classified as either central (with types 1 and 2) or peripheral (type 3, i.e., the involvement of the proximal part of pulmonary arteries; type 4, with the involvement of the proximal part of segmental arteries, and type 5 with the involvement of the terminal part of segmental arteries) disease. Over the past several years, transcatheter implantation has evolved as a safe and reliable treatment for type 3 branch pulmonary artery stenosis (15-17). Two major methods of catheter insertion include BA and SI, with respective success ranges of 53 - 74% and 75 - 86% (17, 18).
In this cross-sectional study, we followed 40 patients with type 3 branch pulmonary stenosis undergoing therapeutic cardiac catheterization (by either BA or SI) for 2 years after the procedure. We found a significant decrease in pulmonary artery pressure gradient immediately after the procedure in both BA and SI groups, which was in accordance with a study conducted by Hiremath et al., who reported a significant decrease in the peak pressure gradient across the stenotic proximal pulmonary artery in 20 patients with unilateral branch pulmonary artery stenosis treated by BA and SI (P = 0.001) (4). The recent research was a multi-center prospective cohort study on patients with biventricular circulation, where the researchers observed 78% angiographic improvement in the diameter of stenotic vessels and a significant decrease in the peak instantaneous pressure gradient across stenotic vessels evidenced by trans-thoracic echocardiography early after catheterization. Finally, they recommended both methods to be similarly effective in managing this type of stenosis.
Geggle et al., in a cohort study on 25 patients with Williams syndrome and peripheral pulmonary artery stenosis, declared successful initial pulmonary artery dilation and a 51% success rate after BA in their patients, who had a mean age of 1.5 years and a mean weight of 9.5 kg, while they expected that serial dilation might be required to resolve stenosis (7). Cunningham et al. (6), in a study to assess the effect of branch pulmonary artery balloon angioplasty on 69 patients with severe isolated pulmonary artery stenosis, reported that the RV/aortic pressure ratio decreased by ≥ 0.3 in 20 patients (31%) and either increased or decreased by ≤ 0.1 in 24 patients (38%). A recent study was conducted on patients with age of ≤ 5 years old, in which those with higher RV-aortic pressure gradient ratios had better outcomes (6).
Nowadays, low-profile new-generation stents have been manufactured, which can be used in children and for redilation up to the diameter of adult vessels. These stents pass through the lower French delivery systems and do not slip or move easily on the balloon when passing through the sheath, and their insertion is safer than previous stents. Therefore, stenosis in underweight patients, which was previously only resolvable by using a balloon, can nowadays be treated with these stents; however, they must be redilated with body growth (18).
According to Figure 2, residual stenosis in patients undergoing BA continuously decreased for up to six months, then gradually increased but did not reach the previous stenotic level. Our study showed that patients weighing lower than 10 kg in the BA group had better outcomes in comparison with their peers weighing over 10 kg. Although 3 children with weight < 10 kg developed moderate to severe restenosis requiring a second balloon angioplasty, their 2-year follow-up showed less restenosis compared to patients weighing > 10 kg (P = 0.049).
Re-stenosis following BA has been reported in 25 - 35% of infants, which is twice as much as the rate observed in our study. As some studies have reported, the best outcomes following BA are seen in patients with discrete stenosis; however, our patients who required a second balloon dilation suffered from long-segment stenosis. Cutting balloons can be used especially for treating long-segment stenoses, and BA results improved from about 50 - 60% success when using standard balloons to about 80 % with cutting balloons. The use of cutting balloons is indicated when stenosis is not resolvable using standard balloons and may be associated with adverse effects such as tearing of the vessel, bleeding, and mortality. The largest available diameter of cutting balloons is 8 mm, limiting its utilization to vessels with a diameter of less than 8 mm.
In our study, the rate of restenosis was lower in the SI group during the one-year follow-up, but stent stenosis increased afterward, which might be due to an increase in the body surface area in comparison with stent lumen diameter or/and neointimal proliferation. This highlights the need for long-term follow-up and further research to fully understand the durability of stent therapy in pediatric patients with pulmonary artery stenosis.
5.1. Limitations
Although our sample size was similar to previous studies, it is recommended to conduct multicenter studies and meta-analyses with larger numbers of patients to achieve more valid results. Long-term follow-up of patients can further illuminate the durability of using balloons and stents, especially among patients weighing less than 10 Kg with discrete stenosis.
5.2. Conclusions
Balloon angioplasty had an immediate success rate as stent implantation in children with branch pulmonary artery stenosis, especially in those with weights less than 10 kg.
Considering that the use of stents in patients weighing less than 10 kg will definitely require several re-dilations in the coming years, it may be recommended to use BA in patients with discrete stenosis and SI in the case of stenosis recurrence.