1. Context
The pigeon environment contains feather dust (bloom), pigeon droppings (guano), and dust from grain feed. Exposure to these elements can lead to the development of diseases such as extrinsic allergic alveolitis/hypersensitive pneumonitis (EAA/HP) (1), Orthosis, and Asthma. Hypersensitive pneumonitis is caused by inhaling immunogenic dust from various sources, including fungi and bacteria. Symptoms during the acute phase include shortness of breath without wheezing, aching discomfort, pain in joints and muscles, and malaise. In the chronic phase, individuals may experience chronic cough and persistent shortness of breath, which can lead to allergic asthma.
In cases of allergic asthma, there is an increase in immunoglobulin E (IgE) levels and positive skin prick tests (2). This condition involves the blocking of receptors on mast cells and basophils, leading to the enhancement of CD23. In the acute phase, symptoms may include cough, fever, chills, and shortness of breath, potentially progressing to a chronic phase with persistent lung inflammation, fibrosis, and irreversible damage resembling chronic allergic asthma and atopic dermatitis (AD).
Effective treatments for EAA/HP due to pigeon allergies include:
- Corticosteroids: Oral or inhaled corticosteroids (ICS) are standard to reduce lung inflammation and control symptoms in both acute and chronic phases.
- Immunosuppressive agents: Severe cases may require immunosuppressive medications like azathioprine or mycophenolate mofetil to suppress the immune response and inflammation.
- Monoclonal antibodies: Therapies such as omalizumab can lower IgE levels, mitigating allergic reactions by blocking FceRI and CD23 receptors.
- Supportive care: Oxygen therapy may be necessary for patients with significant lung damage and impaired oxygen exchange.
Combining these treatments can enhance symptom management and prevent disease progression.
1.1. The Mechanism of Inflammation in the Allergic Alveoli's/Hypersensitive Pneumonitis (EAA/HP)
Exposure to pigeon allergens involves an immune response mediated by IgE. In classic allergic asthma, individuals produce IgE in response to foreign proteins such as those found in pigeon allergens. The tendency to produce IgE is partly influenced by genetic factors, and asthma often co-occurs with other allergic conditions within families, such as allergic rhinitis, eczema, and food allergies (3). Once IgE is produced, it interacts with high-affinity receptors called FCER-1, located on mast cells in the airway mucosa (4).
Upon re-exposure to the allergen, the mast cells release stored mediators from granules and synthesize and release additional mediators. These include histamine, tryptase, leukotrienes C4, and prostaglandin D2. The release of these substances causes smooth muscle contraction and vascular leakage, leading to acute bronchoconstriction known as the "early asthmatic response" (5).
Following the early response, a second phase called the "late asthmatic response" occurs within 3 - 6 hours (6). During this phase, inflammatory cells infiltrate the bronchial mucosa, and bronchial reactivity increases. Cytokines, particularly IL-5, IL-9, and IL-13 produced by lymphocytes, including inner lung lymphocytes, play a significant role in this late response. Cytokines attract and activate eosinophils, stimulate the production of IgE by B lymphocytes, and induce mucus production by bronchial epithelial cells. The exact source of the mediators responsible for the late inflammatory response, whether lymphocytes or mast cells in the airway mucosa, remains unclear. However, corticosteroid therapy is beneficial in asthma treatment as it inhibits the output of pro-inflammatory cytokines within the airways while simultaneously decreasing the responsiveness of airway epithelial cells to cytokines (7).
The conventional understanding of asthma as an allergic disease applies only to a specific subgroup of asthma patients who show evidence of allergies. Allergic asthma is more common in childhood but less prevalent in adults compared to other forms of asthma (8). The current allergen challenge model does not fully explain all aspects of the condition, even in individuals with allergic asthma. Asthma's pathogenesis involves various pathways and mechanisms beyond the production of IgE antibodies and activation of mast cells. Many asthma attacks are not triggered by the inhalation of allergens. However, IgE production and its degree of activation generally correlate with the clinical severity of the disease and are considered fundamental in asthma's pathogenesis due to their presence in the majority of asthma patients.
The mechanisms underlying bronchial hyperreactivity (9), although not completely understood, appear to be associated with inflammation of the airway mucosa. Inhaled corticosteroid treatment, known for its anti-inflammatory effects, is effective in preventing the rise in bronchial reactivity linked to the late asthmatic response. Bronchoconstriction resulting from bronchospasm is not solely caused by the activation of neural pathways; it is also influenced by the effects of released mediators. Muscarinic receptor antagonists, which do not directly affect smooth muscle contractility, have been found to inhibit bronchoconstriction induced by allergen inhalation and airway irritants.
This suggests the involvement of neural pathways in bronchoconstriction. According to the conceptual model proposed, asthmatic bronchospasm results from a combination of mediator release and exaggerated responsiveness to their effects. This indicates that different drugs with various mechanisms of action could effectively treat asthma. For example, bronchospasm triggered by allergen exposure could be reversed or prevented through various medications. These medications work by reducing the amount of IgE bound to mast cells (via anti-IgE antibodies), decreasing the number and activity of eosinophils in the airway mucosa (through anti-IL-5 antibodies), blocking receptors for IL-4 and IL-13 (using anti-IL-4a receptor antibodies), preventing mast cell degranulation (with medications such as cromolyn or nedocromil (10), sympathomimetic agents, and calcium channel blockers), and inhibiting the action of released products (such as antihistamines and leukotriene receptor antagonists). Additionally, asthma treatment can also involve drugs that interfere with the action of inflammatory cytokines (such as anti-IL-5 and anti-IL-13 monoclonal antibodies). Certain medications effective in all forms of asthma include those that relax airway smooth muscle (like sympathomimetic agents and phosphodiesterase inhibitors) or inhibit the effects of acetylcholine released from vagal motor nerves (known as muscarinic antagonists or anticholinergic agents).
Another therapeutic approach in asthma management aims to reduce bronchial responsiveness, as increased airway sensitivity contributes to asthma symptoms. This increased responsiveness is associated with airway inflammation, a characteristic of latter asthmatic responses. Key strategies in managing asthma include minimizing contact with allergens that provoke inflammation and incorporating the use of anti-inflammatory agents as part of a sustained treatment approach, especially ICS. While ICS was initially believed to be effective for all forms of asthma, it is now recognized to be particularly effective for allergic asthma compared to non-allergic asthma (11).
2. Results
The Th1/Th2 balance plays a critical role in asthma. Th1 and Th2 cells are T-helper cells involved in the immune response. In asthma, Th2 cells tend to dominate, producing cytokines that lead to inflammation and allergic reactions, contributing to asthma symptoms. Th1 cells produce cytokines that counteract inflammation. An imbalance favoring Th2 cells can cause chronic inflammation and airway hyperreactivity, typical of asthma.
Th1 cells produce cytokines such as interferon-gamma (IFN-gamma) and interleukin-2 (IL-2), promoting cell-mediated immunity and aiding in clearing intracellular pathogens. In asthma, a weaker Th1 response might reduce the ability to control viral infections and fail to suppress Th2 responses effectively, contributing to airway inflammation. Th2 cells produce cytokines like interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13), which promote allergic reactions, eosinophil recruitment, mucus production, and IgE antibody production. An exaggerated Th2 response in asthma can result in airway inflammation, bronchoconstriction, mucus overproduction, and airway remodeling.
Asthma often involves a dominance of Th2 responses over Th1 responses, leading to chronic inflammation, airway hyperresponsiveness, and the characteristic symptoms of the disease. This imbalance can be influenced by genetic factors, environmental allergens, and respiratory infections. The interaction between Th1 and Th2 responses in asthma and lung inflammation is complex, involving a network of cytokines, immune cells, and regulatory molecules. Maintaining a balance between these responses is crucial for respiratory health and effective asthma management.
The distinction between Th1 and Th2 endotypes is important in understanding asthma. Th2-high asthma is characterized by the activation of mediators such as IL-25 and IL-33, which subsequently activate IL-4, IL-5, IL-13, and thymic stromal lymphopoietin (TSLP), along with non-interleukin-dependent factors (3). This leads to inflammation due to exposure of the airway epithelium to inhaled allergens, microbes, and pollutants, encompassing both allergic and non-allergic inflammation. Approximately half of all asthma patients experience this type of inflammation. The effects of these mediators result in inflammatory cell activation, secretion of IgE, and involvement of the airway epithelium and smooth muscle. Th1-high asthma, on the other hand, is less well understood. It is characterized by patients who are less responsive to corticosteroids, exhibit fewer allergic symptoms, and are typically diagnosed later in life.
In newborn infants, the T-cell population in cord blood tends to exhibit a Th2 phenotype, characterized by a diminished production of IFN-γ (12). This imbalance between Th1 and Th2 cells during the neonatal phase has been associated with an increased risk of developing allergic diseases and asthma. To restore balance and promote proper immune system and lung development in high-risk infants, it has been suggested to expose them to stimuli that enhance Th1-mediated responses. This can include exposure to infections such as Mycobacterium tuberculosis, measles virus, helminths, and hepatitis A virus, as well as endotoxin exposure, increased contact with infections through older siblings, and early daycare attendance. However, factors that disrupt the balance between Th1 and Th2 cells include the frequent use of oral antibiotics, which can alter gut flora. Other factors that promote the Th2 phenotype include living in an industrialized country, exposure to urban environments, dietary factors, and sensitization to house dust mites. Additionally, eosinophilic airway inflammation/hypersensitivity pneumonitis (EAA/HP) can contribute to the Th2 phenotype (13).
2.1. Treatment with Omalizumab
Omalizumab, the first biologic therapy option, is a monoclonal anti-IgE antibody now accessible for individuals with severe asthma. Clinical observations have shown that treatment with omalizumab can significantly enhance the quality of respiratory function in patients, while also decreasing the occurrence of asthma symptoms and severe exacerbations, particularly in individuals with severe allergic asthma. However, the response to omalizumab treatment may vary among patients due to the heterogeneity of severe asthma cases. Therefore, it is crucial to identify biomarkers and clinical features that can distinguish responders from incomplete responders, enabling the effective use of omalizumab in the management of severe asthma. Additionally, certain questions pertaining to the ideal patient population and duration of omalizumab treatment still remain unanswered. This chapter aims to provide an explanation of how omalizumab demonstrates its efficacy in addressing severe allergic asthma and its associated comorbidities through its mechanisms of action and effectiveness.
2.2. Omalizumab Mechanisms of Action
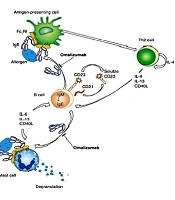
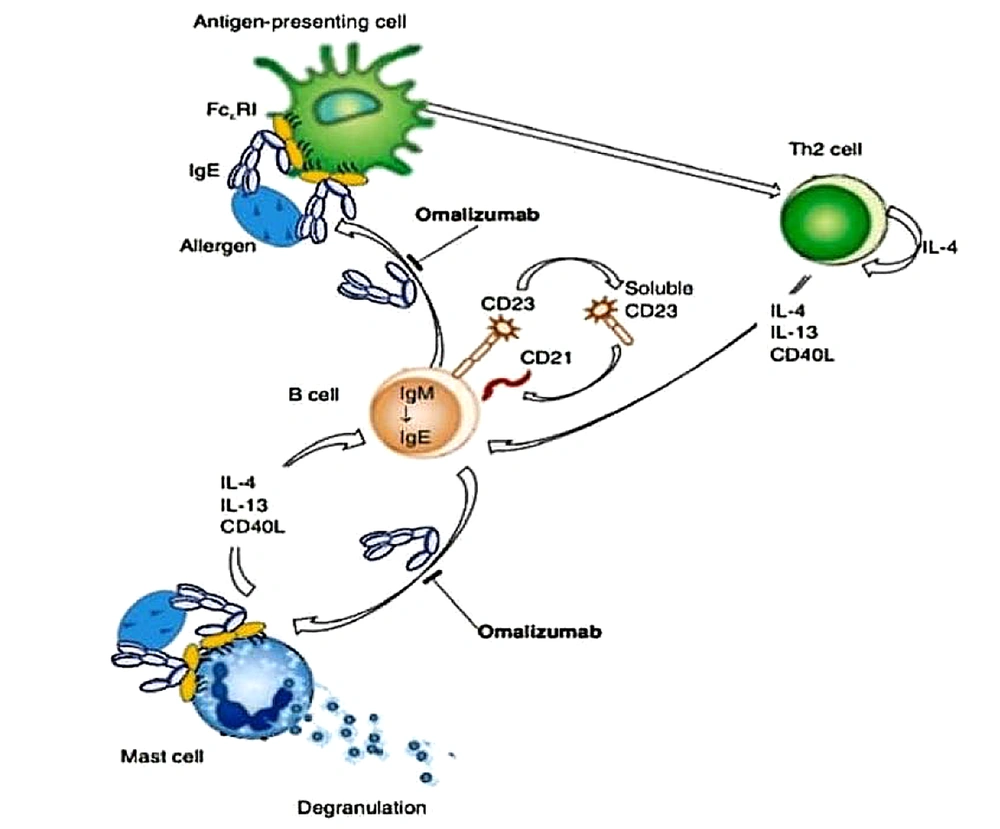
Omalizumab, a humanized anti-IgE monoclonal antibody, is designed to specifically target the Cε3 region of the Fc fragment of IgE. By doing so, it blocks the interaction between circulating IgE and the high-affinity receptors (FcεRI) found on mast cells, basophils, and dendritic cells (DCs), as well as the low-affinity receptors (FcεRII or CD23) present on lymphocytes and eosinophils. These receptors are essential for maintaining IgE balance in the body. By inhibiting the binding between free IgE and FcεRI, omalizumab effectively prevents the release of various inflammatory mediators, including histamine, leukotrienes, and prostaglandins, from mast cells. This mechanism is depicted in Figure 1 (14).
Mechanisms of action of omalizumab, based on a review by Gould and Sutton (14)
Omalizumab has the unique property of not binding to IgE molecules already attached to FcεRI receptors on mast cells or basophils. This characteristic prevents the initiation of allergic reactions. Apart from its direct impact on allergic reactions, omalizumab also plays a crucial role as a disease modifier by reducing the expression of FcεRI receptors and inhibiting IgE production (2). Studies have observed a decrease in IgE-positive cells in the airway tissues of patients treated with omalizumab, regardless of their atopic status (15). Additionally, the expression of FcεRI receptors on mast cells and basophils has been found to be reduced with omalizumab treatment (16).
Omalizumab hinders the binding of free IgE to FcεRI receptors on DCs, consequently impeding the presentation of allergens from DCs to Th2 cells, thereby reducing IgE production by B cells (Figure 1) (14). Furthermore, a study demonstrated that omalizumab decreases IgE production in B cells stimulated with IL-4 and CD40 ligand (17), while another study revealed that it reduces IgE production in B cells by reducing the levels of germline Cε and IL-4R mRNA, without affecting CD23 (18).
2.3. Role of Omalizumab in Allergic Asthma
Omalizumab has obtained global approval for the management of severe or moderate to severe allergic asthma in adults and children aged 12 years or older. The administration of omalizumab involves subcutaneous injections given either every 2 or 4 weeks. The dosage of omalizumab is determined based on the patient's weight and serum IgE levels, which can range from 30 to 1500 IU/mL. The dosage itself can vary from 75 to 600 mg. omalizumab exhibits various effects, as outlined in reference (19).
Research has demonstrated several positive outcomes associated with omalizumab treatment, including improvements in quality of life and pulmonary function, in addition to a reduction in asthma symptoms, asthma exacerbations, and the need for oral and ICS. A meta-analysis of 8 randomized controlled studies indicated that omalizumab led to a significant 43% decrease in severe asthma exacerbations (20). In a study managed specifically with an Asian population, notable improvements were observed in morning peak flow following 16 weeks of omalizumab treatment. Additionally, there was a substantial 68% decrease in the frequency of clinically significant asthma exacerbations compared to the pre-treatment period (21).
Additionally, a meta-analysis of 25 real-world studies, using the Global Evaluation of Treatment Effectiveness, revealed that 77% of patients with severe asthma experienced significant symptom improvement within 4 - 6 months of initiating omalizumab treatment (22). These findings highlight the efficacy of omalizumab in managing severe asthma and its positive impact on patient outcomes.
2.4. Efficacy of Long-Term Omalizumab Use
Omalizumab received its initial approval in Australia in 2002, followed by subsequent approvals in the USA in 2003, the European Union in 2005, and Japan in 2009. The efficacy of long-term omalizumab use has been studied and demonstrated in these regions. Consequently, there is a significant body of evidence available regarding the long-term use and effectiveness of omalizumab treatment.
A study was conducted to assess the effectiveness of omalizumab based on the duration of treatment, and it found no significant differences in asthma control after 4 - 6 months, 12 months, and 24 months of omalizumab use. The study concluded that the efficacy of omalizumab remains consistent even after long-term use. Another study conducted by Sposato et al. demonstrated that patients who underwent omalizumab treatment for a duration of 5 years or more were able to achieve significant reductions in their asthma medications compared to those who had shorter treatment periods (23). Specifically, there was a significant reduction in the use of long-acting beta agonists, montelukast, and oral corticosteroids when the treatment was administered.
2.5. Limitations and Potential Adverse Effects of Omalizumab
2.5.1. Limitations
(1) Cost: Omalizumab is expensive, which can be a significant barrier for patients without adequate insurance coverage.
(2) Route of administration: It is administered via subcutaneous injection, requiring visits to a healthcare provider, which can be inconvenient.
(3) Patient eligibility: It is only indicated for patients with a specific level of serum IgE and who have failed to respond to standard treatments.
(4) Delayed onset: The therapeutic effects of omalizumab can take weeks to months to become evident, which might not be suitable for patients needing immediate relief.
(5) Monitoring Requirements: Regular monitoring is required, including observation for potential allergic reactions post-injection.
2.5.2. Adverse Effects
(1) Injection site reactions: Pain, swelling, redness, and itching at the injection site are common.
(2) Anaphylaxis: A rare but serious allergic reaction that can occur after administration. Patients are usually monitored for a period after injection.
(3) Infections: Some patients may experience an increased risk of upper respiratory infections and sinusitis.
(4) Headache: One of the most commonly reported side effects.
(5) Arthralgia: Joint pain is reported by some patients.
(6) Gastrointestinal Problems: Some patients report nausea, abdominal pain, and diarrhea.
(7) Cardiovascular issues: Rare cases of cardiovascular and cerebrovascular adverse events have been reported.
(8) Malignancy: There is a theoretical concern about the potential for an increased risk of malignancy, although studies have not conclusively proven this link.
3. Conclusions
Extrinsic allergic alveolitis (EAA), also known as Hypersensitivity Pneumonitis (HP), is an inflammatory syndrome affecting the lungs, caused by an immune response to inhaled environmental antigens. Effective management of EAA/HP involves several strategies:
(1) Antigen avoidance: Identifying and avoiding the causative antigen is paramount to managing EAA/HP. This involves environmental control measures and lifestyle changes.
(2) Pharmacotherapy: Corticosteroids are the primary treatment for acute exacerbations and severe cases. Long-term use is limited due to side effects, necessitating the need for alternative treatments.
(3) Monitoring and Follow-up: Regular follow-up with pulmonary function tests and imaging is crucial to assess disease progression and treatment efficacy.
(4) Immunosuppressive therapy: In chronic or refractory cases, immunosuppressive agents like azathioprine or mycophenolate mofetil are considered.
Omalizumab, an anti-IgE monoclonal antibody, is primarily used for treating allergic asthma and chronic idiopathic urticaria. Emerging evidence suggests its potential efficacy in managing EAA/HP, particularly in cases associated with elevated IgE levels and allergic mechanisms. These combination therapies aim to improve efficacy, decrease steroid reliance, and enhance patient outcomes:
(1) Omalizumab and corticosteroids: Initial studies indicate that combining omalizumab with corticosteroids may allow for lower steroid dosages while maintaining symptom control, which is particularly advantageous for patients with chronic or severe conditions.
(2) Omalizumab and immunosuppressive agents: Some case reports and small studies suggest that this combination may be effective in refractory cases where traditional treatments fail, though larger controlled trials are needed to validate these results.
(3) Omalizumab and bronchodilators: Evidence suggests that patients receiving both treatments experience improved lung function and symptom control, indicating a complementary mechanism of action.
Omalizumab shows potential not only as a standalone therapy but also as part of combination treatments for allergic and inflammatory diseases like EAA/HP. Its capacity to lower IgE levels and modulate the immune response makes it an important tool in personalized medicine. Further research, especially large-scale clinical trials, is essential to determine optimal combination therapies and fully understand the long-term benefits and safety of these treatment strategies.