1. Introduction
Elizabethkingia meningoseptica (EM), previously called Flavobacterium meningosepticum, is a gram-negative, non-motile, non-fermentative, oxidase-positive, rod-shaped bacterium from the genus of the same name (Elisabethkingia). It was first described by Elizabeth O. King in 1959 (1). It is ubiquitous and present in the environment in water and soil. It can be isolated from tap water (even when treated with chloride), medical and other equipment (e.g., catheters, isolettes, sondes, and air conditioners), and even some disinfectants. As the name suggests, the bacterium causes meningitis and sepsis, especially in the pediatric population. Still, it can cause severe infections in the elderly because this age group is more susceptible to certain risk factors (2). It is a rare, atypical bacterium that usually causes nosocomial infections and rarely community-acquired infections. It can be brought into connection with patients who were previously hospitalized, connected to different devices, and have an altered immune status. This agent causes severe infections, most commonly neonatal meningitis, in immunocompromised patients and preterm newborns (3). Other than meningitis, it can cause infections of other organs, such as osteomyelitis, skin lesions, endocarditis, sinusitis, and pneumonia.
This infection in immunocompromised patients is complicated by the fact that this strain is usually multiresistant, especially to beta-lactams, carbapenems, and aminoglycosides, and treatment can be a challenge (EM produces two different types of beta-lactamases) (4). Vancomycin, rifampicin, fluoroquinolones of the newer generation, piperacillin-tazobactam, minocycline, and tigecycline can be used for this infection (5). Combined therapy has shown better results than monotherapy. However, additional studies should determine an optimal treatment combination (4).
Elizabethkingia meningoseptica shows an unpredictable antimicrobial resistance, which depends on the isolation region. Therefore, it would be of the utmost importance to show its extent and antimicrobial resistance from different parts of the world, which can shine a light on the bacterium’s behavior. Having all this in mind, inadequate and untimely therapy can result in high mortality and complications.
2. Case Presentation
A sixteen-day-old newborn was admitted to the Institute for Child and Youth Healthcare of Vojvodina. Hetero-anamnestically, one day before admittance, he presented a fever of up to 39.4°C rectally, started fussing, and refused to eat. He was a preterm newborn, born as a second twin from the first pregnancy conceived naturally. Born via Caesarean section at 34 weeks and 1 day gestation, with an Apgar score of 8/9, weighed 2500 grams, and was 46 cm tall. Positioned longitudinally, with occipital presentation and clear amniotic fluid on birth. After birth, it showed the clinical signs of mild respiratory distress syndrome, and nasal continuous positive airway pressure was applied. After the first day, he did not need oxygenic support. There were no signs of infection in the perinatal and early neonatal period. He was breastfed combined with the premature formula.
On admittance, the 16-day-old newborn was afebrile and had been previously given an antipyretic. Oxygen saturation was 94% in the room air, with respirations of 45/min and pulse of 156/minute. Arterial tension was 80/50 mmHg. He was adynamical with asynchronous spontaneous activity and had a dyskinetic bulbomotor activity. Crying was monotonal. The skin was pale and marmorized with peripheral acrocyanosis. He was dystonic with incomplete atavistic reflexes and variable muscle tone.
Laboratory tests were completed, showing high C reactive protein (162.9 mg/l), leukocytosis with neutrophil predominance, and compensated metabolic acidosis. High lactate levels (3.2 mmol/l) and hyponatremia (120 mmol/l) were recorded. The neonate was admitted to the Department of Neonatal Intensive Care Unit. Hemoculture was sampled, and a lumbar puncture was performed to evaluate for septicemia and meningitis. Cerebrospinal fluid (CSF) was cloudy, and cytological and biochemical analyses indicated bacterial meningitis.
Clinical presentation, high fever with extremely high inflammation markers, leucocytosis, and high neutrophil count indicated a bacterial infection, which, together with CFS findings, were sufficient for starting antibiotic therapy. Initially, meropenem and vancomycin were administered because vancomycin combined with an antibiotic from another group is an empirically chosen drug regimen for treating late-onset sepsis. After two days, the results of bacterial tests showed that EM was isolated from the blood (the analysis was completed using BD BACTEC FX40 automated blood culture system, and identification was by matrix–assisted laser desorption ionization–time–of–flight mass spectrometry) and CSF. The analyses were conducted in the clinical microbiology department of the national laboratory. Before replacing the antibiotics, another set of cultures (from blood and CSF) was performed on the third day of treatment, and the same results were obtained. The antibiogram showed resistance to carbapenems, piperacillin-tazobactam, cephalosporins, aminoglycoside, and polymyxin. Therefore, antibiotic treatment was corrected, replacing meropenem with trimethoprim-sulfamethoxazole and levofloxacin. On the mentioned therapy, administered for three weeks, the neonate became afebrile, with an improvement in clinical findings. After three weeks, feverishness reoccurred, followed by altered neurological findings. Cultures were performed again, showing sterile results, but CSF analysis showed pleocytosis. In ultrasonography, periventriculitis was observed with early signs of hydrocephalus. As a result, trimethoprim-sulfamethoxazole was replaced with ciprofloxacin.
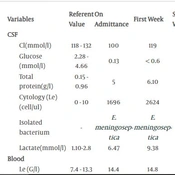
Laboratory findings during the treatment are shown in Table 1.
| Variables | Referent Value | On Admittance | First Week | Second Week | Third Week | Fourth Week | Fifth Week | Sixth Week |
|---|---|---|---|---|---|---|---|---|
| CSF | ||||||||
| Cl(mmol/l) | 118 - 132 | 100 | 119 | 119 | 120 | 123 | 121 | 121 |
| Glucose (mmol/l) | 2.28 - 4.66 | 0.13 | < 0.6 | 1.24 | 1.44 | 1.65 | 2.03 | 2.3 |
| Total protein(g/l) | 0.15 - 0.96 | 5 | 6.10 | 2.9 | 1.65 | 1.58 | 1.49 | 0.58 |
| Cytology (Le)(cell/ul) | 0 - 10 | 1696 | 2624 | 173 | 92 | 63 | 52 | 3 |
| Isolated bacterium | - | E. meningoseptica | E. meningoseptica | - | - | - | - | - |
| Lactate(mmol/l) | 1.10-2.8 | 6.47 | 9.38 | 3.90 | 2.80 | 2 | 1.45 | 1.13 |
| Blood | ||||||||
| Le (G/l) | 7.4 - 13.3 | 14.4 | 14.8 | 17.9 | 24 | 5.4 | 10.7 | 8.4 |
| Neutrophil (%) | 18.4 - 32.4 | 59.8 | 43.5 | 27.6 | 54.5 | 60.3 | 32.8 | 22.2 |
| Lymphocyte (%) | 39.8 - 60.6 | 22.6 | 41.3 | 51.3 | 29.3 | 25.2 | 48.2 | 66.1 |
| Monocyte (%) | 7.7 - 13.8 | 17 | 11 | 13.7 | 11.7 | 10.7 | 12.5 | 8.6 |
| Glucose(mmol/l) | 2.7 - 4.4 | 5.9 | 4 | 3.9 | 4.1 | 4.2 | 4.7 | 4.2 |
| CRP(mg/l) | 0 - 5 | 162.9 | 126.3 | 52.6 | 2.8 | 50.2 | 0.6 | 0.9 |
| Lactate | 0.5 - 2.2 | 3.2 | 2.09 | 1.53 | 1.52 | 2 | 2.1 | 1.9 |
| Isolated bacterium | - | E. meningoseptica | E. meningoseptica | - | - | - | - | - |
| IgA | 0.1 - 1.31 | 0.06 | 0.1 | 0.04 | 0.03 | 0.1 | ||
| IgM | 0.4 - 1.4 | 0.53 | 0.42 | 0.41 | 0.42 | |||
| IgG1 | 1.94 - 8.42 | 1.38 | 1.4 | 1.20 | 1 | 1.7 | ||
| IgG2 | 0.225 - 3 | 0.63 | 0.58 | 0.69 | ||||
| IgG3 | 0.186 - 0.9 | 0.27 | 0.29 | 0.24 | ||||
| IgG4 | 0.005 - 0.8 | 0.01 | 0.01 | 0.03 |
Biochemical and Cytological Blood and CSF Findings During Hospitalization
During hospitalization, therapy was corrected on several occasions following antibiogram and bacterial resistance. Blood was sampled for culture on admittance, after the initial antibiotic therapy, and every 24 and 48 h after replacing antibiotics. Antimicrobial therapy was administered for 6 weeks in total. During the first week of therapy, magnetic resonance imaging (MRI) showed white mass edema and left transverse sinus thrombosis, after which low-molecular-weight heparin was given. The results of electrocardiography and echocardiography were normal. Other results did not show the signs of infection in other organs.
When immunological analysis was performed, it showed very low levels of immunoglobulin (Ig) A and low levels of IgG subclasses. After consultation with an immunologist, intravenous Ig (IVIG) was administered, which, combined with ciprofloxacin therapy, led to the normalization of CSF findings and ensured a well-favored therapy effect. The IVIG substitution therapy was repeated after four weeks.
Neurological examination was regularly performed during hospitalization. The newborn was evaluated and monitored with different imaging methods (brain ultrasonography and MRI). Regular neuroradiologic imaging follow-ups were necessary because of alterations in neurological findings. On the 23rd hospital day, an MRI showed the dilation of the ventricular system and the development of triventricular hydrocephalus as a result of an adhesion in Sylvian aqueduct. The previously described thrombotic mass was almost entirely recanalized. The newborn had a ventriculoperitoneal shunt implanted in the department of neurosurgery. After the procedure, the ventricular system stopped expanding. The patient was hemodynamically stable and afebrile, with sterile cultures (hemoculture and CSF). He was discharged with recommendations for further physical, immunological, and neurosurgical follow-ups and regular follow-ups by a developmental neurologist.
The twin brother also had an immunodeficiency and started IVIG substitutional therapy. An epidemiological survey and analysis showed no signs of bacterial growth from the swabs of surroundings and other family members, including the twin brother. As the twin was healthy and showed no signs of infections, it was decided not to test him for meningitis. Adequate and timely therapy and control of the epidemiological situation led to the eradication of EM. Therefore, intrahospital spreading was prevented.
3. Discussion
Neonatal meningitis is still an alarming disease, with a high mortality rate ranging from 20% to 60% (6). The authors describe a difference in mortality between developed countries (around 10%) and developing countries (with a rate of 40%-58%). There is also a difference in pathogen specter, which causes neonatal meningitis, between the two countries. The most common causes of meningitis in developed countries are the Streptococcus B group, Escherichia coli, and Listeria monocytogenes (7). In contrast, other gram-negative and positive bacteria come into consideration in other countries. The incidence of EM meningitis is also higher in undeveloped and developing countries compared to developed countries.
Elizabethkingia meningoseptica is a rare cause of disease. The most common infection caused by this bacteria is meningitis. Immunocompromised patients are at the highest risk for developing this disease, which most commonly includes newborns, especially preterm newborns, and adults with malignant diseases or diabetes. The high mortality rate and risk of neurological sequelae, even with current diagnostics and therapeutic modalities, is the reason why neonatal meningitis presents a tough challenge for clinicians, especially in preterm neonates.
Reacting promptly with adequate antibiotic treatment is the key to good outcomes. The decision for the treatment approach of EM meningitis in newborns is demanding because it is highly multiresistant. The sensitivity of this bacterium is seldom predictable (8). Authors often mention the resistance of multiresistant EM strains to ampicillin, ceftazidime, imipenem, gentamicin, ciprofloxacin, and sulfmetoksazol-trimetoprim while pointing out sensitivity to piperacillin and amikacin, with intermittent to good sensitivity to rifampicin and vancomycin (9). In our case, EM was resistant to carbapenems, piperacillin-tazobactam, cephalosporins, aminoglycosides, and polymyxin, while was sensitive to trimethoprim-sulfamethoxazole, levofloxacin, and colomycin.
In contrast to the findings of Güngör et al., which showed an inadequate response to ciprofloxacin therapy (although in vitro sensitivity was confirmed), in our case, a considerable response and improvement were observed after administering ciprofloxacin (10). It is very important to present the antimicrobial resistance of EM and share treatment experiences from different parts of the world because it will improve communication between clinicians and result in being one step closer to making a comprehensive and improved treatment guide.
Regardless of the aggressive approach and treatment promptness, neonatal meningitis carries many complications, such as ventriculitis, hydrocephalus, abscess forming, effusions, and empyema. In our case, triventricular hydrocephalus developed as a consequence of Sylvian duct adhesion. Immunocompromised patients are at the highest risk for developing this disease. In our twin late-preterm newborn, immunodeficiency was diagnosed, which led to a prolonged and complicated clinical course.
Following the epidemiological measures should be one of the priorities because it can prevent the intrahospital spreading of this multiresistance strain. Waterborne transmission is one of the most common spreading methods of this bacterium. Therefore, the epidemiological situation can reach a large scale (11). When the clinical course is troublesome with complications and the disease is prolonged, immunodeficiency should be considered, and additional specific diagnostic modalities should be performed.
3.1. Conclusions
Although neonatal meningitis caused by EM is often described in undeveloped and developing countries, this is rarely the case in Serbia. In our institute, it has not been described for the last 20 years. Publishing this case is important as it can show the presence and resistance of this strain in our country. Considering its high mortality and morbidity rate and its multiresistance, EM requires an early diagnosis and adequate and intensive therapy. The occurrence of severe infections in newborns should arouse the suspicion of immunodeficiency, which requires an immediate diagnosis and treatment.