1. Background
Childhood and adolescent obesity have become a worldwide problem. Excess weight in childhood and adolescence is a significant risk factor for developing obesity and adult comorbidities (1). Obesity and overeating are associated with the accumulation and expansion of adipose tissue. Hypertrophic adipocytes may play an important role in initiating inflammation by disrupting the balance of inflammatory and anti-inflammatory factors (2). There are some studies suggesting that serum 25-hydroxy vitamin D (25 (OH) D) level is associated with obesity (2, 3). Adipose tissue is recognized as an important endocrine organ that secretes adipokines rather than being a passive energy store. Dysregulated production of these adipokines may contribute to the pathogenesis of metabolic disorders, including insulin resistance and type 2 diabetes (4). One of these adipokines is omentin 1. Omentin 1 mainly plays an important role in various processes such as regulating the uptake of long-chain free fatty acids and body fat storage, modulating food intake and energy metabolism, and regulating anti-inflammatory, antidiabetic, anti-atherogenic and insulin-sensitizing effects in the body. Obesity causes changes in the adipose tissue microenvironment, leading to adipose tissue dysfunction and an accompanying state of chronic low-grade inflammation (2). Vitamin D, a pleiotropic hormone retained in adipose tissue, has been shown to affect the production and function of adipokines (4, 5). Omentin is derived from human omental adipose tissue. In addition, it is only known to be secreted from stromal vascular cells of visceral adipose tissue. Studies show that omentin-1 levels are low in obese and insulin-resistant patients (6). Increasing evidence indicates a strong association between vitamin D deficiency and decreased insulin secretion (7). In the literature, there are a limited number of studies investigating the relationship between serum omentin-1 levels and vitamin D in adults (2, 7). Obese adolescents are known to be a risky group regarding vitamin D deficiency (8).
2. Objectives
No study has investigated the relationship between Vit D and omentin-1 in obese adolescents. Our study aimed to evaluate serum omentin 1 level in obese adolescents with vitamin D deficiency.
3. Methods
In this cross-sectional prospective study, we included 83 obese adolescents (age range: 11 - 17 years) referred to the pediatric outpatient clinic (University of Health Sciences Bağcılar Training and Research Hospital, Turkey) between April and September 2020. After the patients’ routine examinations, adolescents with pubertal stage II and above, according to Tanner measurements, and those with a BMI above the 95th percentile were included in the study. The adolescents with infections, endocrine or metabolic diseases, those using dietary supplementation, and those who did not give informed consent were excluded.
After obtaining written consent from the families of the adolescents participating in the study, serum samples were collected in the morning after 10 - 12 hours of fasting. At the same time, blood was drawn into a separate tube to study the omentin 1 level. After collection, serum samples were obtained by incubation at 2 - 8 degrees Celsius for 30 minutes and centrifugation at 1000 rpm for 15 minutes. Serum (25(OH)D) level, fasting blood glucose, insulin, and lipid profile were studied. Serum samples reserved for omentin 1 measurement were stored at -80 degrees Celsius until the time of the study. Total cholesterol (TC), fasting blood glucose (FBG), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were included in routine obesity tests and measured by biochemical enzymatic colorimetric test methods. These biochemical and immune chemical tests were carried out with the Biotek instruments elx800 8000 (Velmont, USA) analyzer. Vit D measurements were made using the human vitamin-D 25-OH ELISA Kit (Shanghai, China Catalog No: E1543H) based on the Bioassay Technologies sandwich-ELISA method. The analytical performance characteristics of this kit included an analytical sensitivity of 5.19 ng/mL, an analytical measurement range of 10 - 2000 ng/mL, and % CV values < 10%.
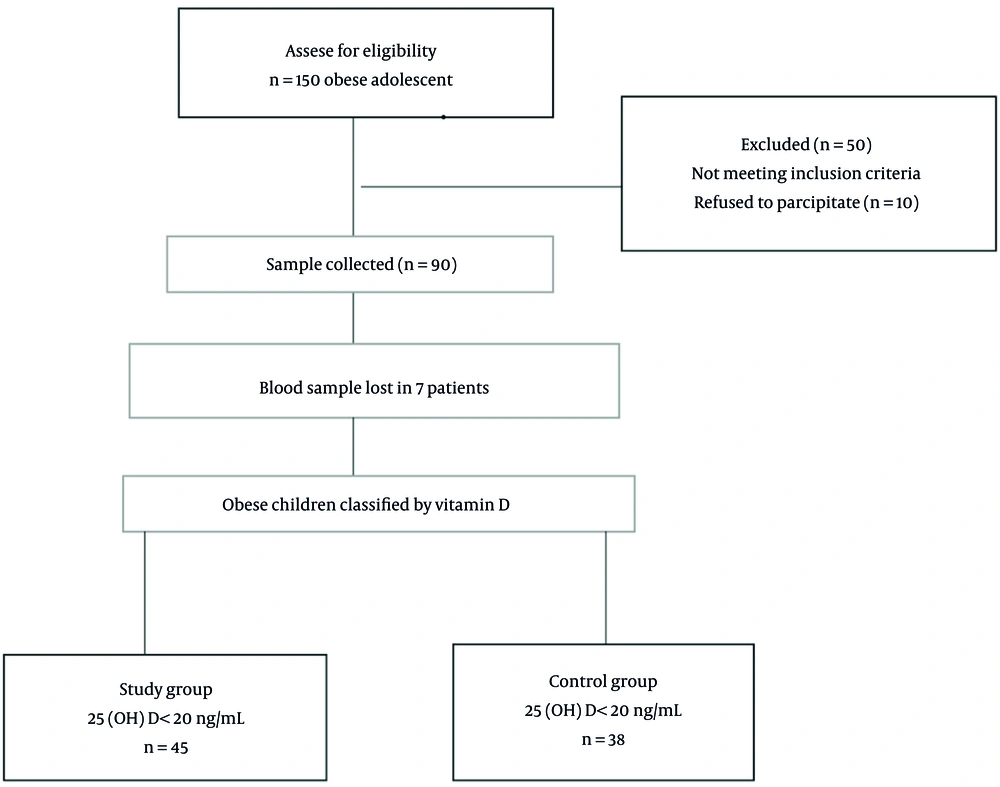
Obese adolescents were evaluated regarding their vitamin D levels. 25 (OH) D level below 20 ng/mL has been accepted as vitamin D deficiency (9). Of the 83 obese patients, 45 with 25(OH)D concentrations below 20 ng/mL were considered the study group, and 38 with 25(OH)D concentrations ≥ 20 ng/mL were considered as the control group.
After the case and control groups were separated according to vitamin D levels, omentin 1 levels were determined in serum samples, which were taken in separate tubes and stored at -80 degrees Celsius.
Omentin-1 measurements were made by utilizing the Human Omentin ELISA Kit (Shanghai, China Catalog No: E3770H) based on the Bioassay Technologies sandwich-ELISA method. The analytical performance characteristics of this kit included an analytical sensitivity of 1.03 ng/mL, an analytical measurement range of 2 - 600 ng/mL, and %CV values < 10%.
The research protocol was approved by the Bağcılar Training and Research Hospital Research Ethics Committee following the Declaration of Helsinki (Approval number: 2020.09.1.03.120).
3.1. Statistical Analysis
Data analyses were performed by the NCSS (Number Cruncher Statistical System) 2007 Software (Utah, USA) package. Besides descriptive statistical methods (mean and standard deviation), the distribution of variables was investigated by the Shapiro-Wilk test of normality, and the independent samples t-test was conducted to compare the variables with normal distribution between paired groups. The Mann-Whitney U test was carried out to compare the nonnormally distributed variables between paired groups. The chi-square test was performed to compare qualitative data, and the Pearson correlation test was applied to indicate the correlations between the variables. Linear regression analysis was done with the variables associated with omentin-1. In the differential diagnosis of low Vit D levels in obese children, receiver-operator curve (ROC) analysis was carried out for omentin-1, and the predictive value was calculated. The significance level was P < 0.05.
4. Results
There were 83 patients eligible for this study. Figure 1 describes the enrollment process. The average 25(OH)D value of the study group was 17.14 ± 2.22 ng/mL, and the average 25(OH)D value of the control group was 45.29 ± 24.98 ng/mL. The 25(OH)D levels of girls were 31.24 ± 24.21 ng/mL, and the 25(OH)D concentrations of boys were 31.18 ± 20.93 ng/mL. No significant difference was detected between the average 25(OH)D concentrations between the groups (P = 0.991). Table 1 indicates the laboratory and clinical parameters of the control and study groups. There was no significant difference between the groups in sex, anthropometric measurements, biochemical values, or lipid profile. The mean omentin-1 concentration of the control and study groups was 262.5 ± 136.31 and 113.23 ± 15.98 ng/mL, respectively. The mean omentin-1 concentration of the study group was significantly lower compared to the control group (P = 0.0001) (Table 1).
| Variables | Control Group, 25(OH)D ≥ 20 ng/mL | Study Group, 25(OH)D < 20 ng/mL | P-Value |
|---|---|---|---|
| Age | 13.22 ± 1.69 | 13.62 ± 1.54 | 0.244 b |
| Sex | 0.091 c | ||
| Boy | 25 (55.56) | 17 (37.78) | |
| Girl | 20 (44.44) | 28 (62.22) | |
| BMI (kg/m2) | 29.82 ± 4.16 | 29.86 ± 2.78 | 0.952 b |
| TC (mg/dL) | 175.11 ± 36.17 | 177.42 ± 35.47 | 0.760 |
| LDL (mg/dL) | 113.91 ± 28.09 | 115.52 ± 26.15 | 0.780 |
| HDL (mg/dL) | 43.73 ± 7.82 | 45.88 ± 9.77 | 0.253 |
| TG (mg/dL) | 154.2 ± 103.36 | 119.98 ± 64.49 | 0.063 |
| FBG (mg/dL) | 89.47 ± 8.95 | 91.67 ± 8.88 | 0.245 |
| Insulin (μIU/mL) | 18.14 ± 9.98 | 18.39 ± 9.68 | 0.902 |
| Omentin-1 (ng/mL) | 262.5 ± 136.31 | 113.23 ± 15.98 | 0.0001 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; HDL-C, high-density lipoprotein cholesterol; FBG, fasting blood glucose; BMI, body mass index; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride. The significance of bold values is P < 0.05.
a Values are expressed as Mean ± SD or No. (%).
b Independent samples t-test.
c Chi-square test.
The mean omentin-1 concentration of girls was 186.70 ± 127.92 ng/mL, and the mean omentin-1 level of boys was 189.19 ± 116.96 ng/mL. No significant difference was detected between boys and girls (P = 0.924).
Logistic regression analysis was conducted with omentin-1. Omentin-1 was found to be significant in univariate tests, with an OR (95% CI) of 0.98 (0.89 - 1.01) (P = 0.0001). There was a significant and positive correlation between omentin-1 and 25(OH)D (r = 0.988 P = 0.0001). No significant correlation was determined between omentin-1 and body mass index (BMI), LDL-C, TC, TG, HDL-C, FBG, and insulin variables (P > 0.05) (Table 2). In univariate tests, linear regression analysis was carried out with 25(OH)D and omentin-1, and 25(OH)D displayed a significant positive correlation (P = 0.0001) (Table 3).
| Variables | Omentin-1 |
|---|---|
| 25-OH vitamin D (ng/mL) | |
| r | 0.988 |
| P | 0.0001 |
| Age | |
| r | - 0.146 |
| P | 0.168 |
| BMI kg/m2 | |
| r | - 0.088 |
| P | 0.413 |
| TC (mg/dL) | |
| r | - 0.145 |
| P | 0.171 |
| LDL-C (mg/dL) | |
| r | - 0.125 |
| P | 0.241 |
| HDL-C (mg/dL) | |
| r | - 0.100 |
| P | 0.347 |
| TG (mg/dL) | |
| r | 0.115 |
| P | 0.281 |
| FBG (mg/dL) | |
| r | - 0.013 |
| P | 0.905 |
| Insulin (μIU/mL) | |
| r | 0.079 |
| P | 0.462 |
a Pearson’s correlation test.
| Variable | Unstandardized Coefficients | Standardized Coefficients | t | P-Value | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| 25-OH vitamin D (ng/mL) | 5.34 | 0.09 | 0.99 | 59.21 | 0.0001 |
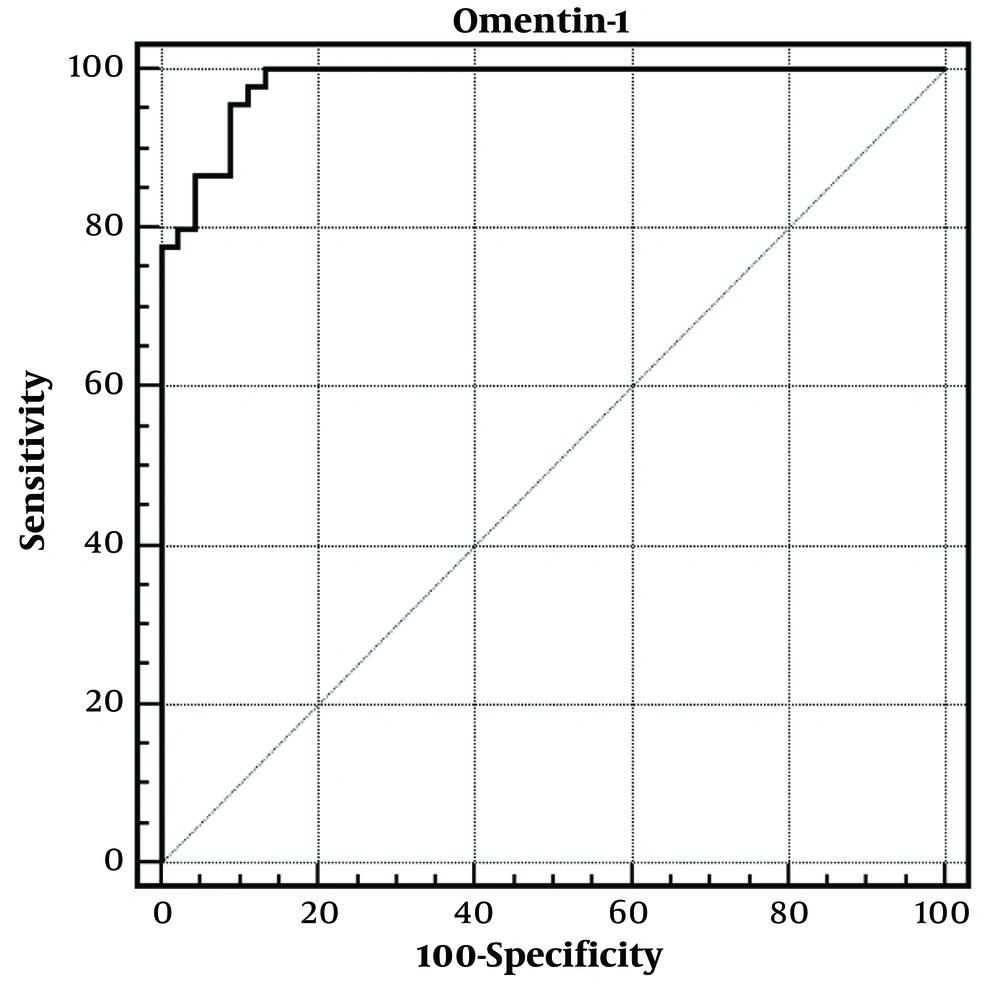
In the differential diagnosis of low vitamin D levels in obese children, ROC curve analysis was performed for omentin-1, and the area under the ROC curve was 0.983 (0.930 - 0.998). For omentin-1 level ≤ 135.01 ng/mL, the specificity was 91.11, sensitivity was 95.56, negative predictive value was 95.3, positive predictive value was 91.5, and LR (+) value was 10.75. A ROC analysis was done to determine the area under the curve and the optimal cut-off point for the serum omentin-1 level to diagnose obese adolescents with vitamin D deficiency. The optimal cut-off point (sensitivity; specificity) for the serum omentin-1 level was 135.01 ng/mL. Thus, obese adolescents with serum omentin-1 levels ≤135.01 ng/mL are 10.7 times more likely to have vitamin D deficiency (Figure 2).
5. Discussion
Our study is the first study investigating omentin 1 levels in obese adolescents with vitamin D deficiency. There is no study evaluating the relationship between vitamin D and omentin 1 in obese individuals. A significant positive correlation was found between vitamin D level and omentin 1 level. In previous studies, serum omentin 1 levels were found to be low in pediatric and adolescent obese populations as well as in adult obese patients (10-14). Omentin 1 is one of the new adipokines investigated concerning obesity and comorbidities of obesity.
As a source of inflammation, adipose tissue is an active, complex metabolic, endocrine organ producing different cytokines. Adipose tissue-derived adipokines regulate inflammation, diabetes, energy metabolism, and atherosclerosis (15). Omentin is an adipocytokine predominantly expressed in visceral omental adipose tissue, and its major isoform in human plasma is identified as omentin-1 (16). The control of omentin 1 within fat tissue is influenced by various factors, and its exact function in glucose energy metabolism remains unclear (17). Laboratory investigations have indicated that omentin 1 improves insulin signaling by initiating the protein kinase Akt/protein kinase B and boosts glucose transportation in isolated human adipocytes, all without acting like insulin. Vitamin D deficiency is observed more frequently in the adolescent age group than in children due to the increasing needs of the growing organism (8, 18). Vitamin D deficiency is more widespread in overweight children and adolescents (8, 19). Some studies performed in recent years have demonstrated a bidirectional correlation between vitamin D and obesity (20). Because of the increased fat mass in obese people, high amounts of vitamin D are retained in the adipose tissues, and the vitamin D level in the serum is low (21). Vitamin D has multifactorial effects, and some of its effects are also seen in adipose tissue. Therefore, changes in vitamin D levels may affect adipokines released from adipose tissue. It is thought that vitamin D may exert some of its systemic effects indirectly through these adipokines (22). Very few studies evaluate the relationship between vitamin D and omentin in the literature. Zorlu et al. reported an inverse relationship between serum omentin levels and vitamin D in women with vitamin D deficiency (23). However, the study of Zorlu et al. was conducted with non-obese women. In our study, serum omentin 1 levels were low in obese adolescents with vitamin D deficiency, and a significant positive correlation was found between serum vitamin D levels and serum omentin 1 concentrations. In our study, an omentin 1 level below the optimal cut point of 135.01 was defined as omentin 1 deficiency in adolescents with low vitamin D levels.
It is established that obese individuals generally exhibit reduced levels of serum omentin 1. Tan et al. demonstrated through both laboratory and real-world studies that omentin 1 production is reduced by both insulin and glucose, whether inside the body or in controlled conditions. Additionally, their research highlighted that women with polycystic ovary syndrome (PCOS) who are overweight or obese have notably lower levels of omentin 1 in their plasma and adipose tissue compared to women without PCOS but with similar body mass index (BMI). This suggests that insulin significantly governs the production of omentin 1 in adipose tissue (17). Studies have shown that vitamin D can modulate the secretion of many adipokines from adipose tissue (5).
In our study, there was no correlation between serum omentin 1 level and BMI. The reason for this was interpreted as the fact that both our study and control group consisted of obese cases. Unlike our study, in the study conducted by Çatli et al. in obese children, serum omentin 1 level was significantly lower in both pediatric and adolescent age groups compared to the control group. In this study, the omentin 1 level showed a negative correlation with BMI in the adolescent age group, while no correlation was found between the omentin 1 level and anthropometric measurements and metabolic parameters in the prepubertal age group (13). In this study, since the obese group was compared with the healthy control group, BMI and omentin 1 level showed a negative correlation. In the study of Büyükinan et al., serum omentin 1 level was significantly lower in obese children with metabolic syndrome (MS) compared to obese children without MS. Unlike our study, serum omentin-1 level was inversely correlated with BMI in obese children with MS (12).
Our study found no correlation between serum lipid profile, insulin, fasting blood glucose, and serum omentin 1 level. This indicated that serum omentin-1 level was more related to inflammation. Catoi et al. reported that omentin-1 levels were lower in adult morbidly obese patients compared to the normal weight control group (24). Similar to our study, no correlation was found between insulin or lipid panel and omentin-1 levels in this study. Catoi et al. reported that omentin-1 was inversely correlated with TNF-α and explained that decreased omentin-1 levels are associated with chronic inflammation in obesity. Obesity is associated with insulin resistance and chronic inflammation, but omentin-1 appears to be a positive marker in preventing obesity-related disease development (24). Similar to our study, Büyükinan et al. found that omentin-1 levels were not associated with insulin resistance, lipids, and abnormal glucose metabolism in obese children with MS (12).
In the study by Türkkan et al., serum omentin 1 level was lower in obese adolescents with non-alcoholic fatty liver disease (25). They found no relationship between serum omentin 1 level and BMI and other lipid profiles in obese adolescents without non-alcoholic fatty liver disease.
Our study found no significant difference between male and female adolescents regarding omentin-1 levels. Such a difference has not been found in the literature. Oswiecimska et al., in their study including anorexia nervosa, obese, and healthy control group, found that serum omentin-1 level was low in the obese population. However, the study was conducted only in female adolescents (26). We think that the reason why there was no difference in our study was because their BMIs were similar. Increasing evidence points to a strong association between vitamin D deficiency and decreased insulin secretion in both human and animal models (7). Given the modulatory and anti-inflammatory effects of vitamin D, Cheshmazar et al. hypothesized that a low-calorie diet program combined with vitamin D supplementation might improve serum omentin-1 levels and modulate inflammatory markers, lipid profiles, and anthropometric parameters in overweight and obese individuals. As a result of this study, vitamin D supplementation for eight weeks in combination with a low-calorie diet program significantly reduced inflammatory markers in obese individuals but did not change serum omentin-1 concentration (2). In this study, it was thought that vitamin D might have a positive effect, but no increase in omentin 1 level was observed in the result. But it was still observed that vitamin D supplementation provided a significant decrease in inflammation parameters. Our study shows that vitamin D has a modulatory and anti-inflammatory role on adipose tissue in accordance with other studies in the literature. As a result, it explains the relationship between vitamin D and omentin 1 in inflammation in obesity.
In addition, based on the results of our study, omentin 1 level may be used as an early marker to detect vitamin D deficiency in the early stage of inflammation in obesity before metabolic deterioration.
Our study is the first to evaluate the correlation between serum omentin-1 levels and vitamin D deficiency in obese adolescents. This feature is the strength of our study. In our study, omentin-1 levels were assessed only in individuals with a BMI above the 95th percentile among obese individuals with low vitamin D levels. It would be better if obese children were classified among themselves as overweight, obese, and morbidly obese. This is a weakness of our research.
5.1. Conclusions
Our study observed that the omentin 1 level was also low in obese adolescents with vitamin D deficiency, and there was a significant positive correlation between vitamin D and the omentin 1 level. As a result, it explains the relationship between vitamin D and omentin 1 in inflammation in obesity. Serum omentin-1 can be employed as a biomarker in obese adolescents with vitamin D deficiency.