1. Background
Seizure is one of the most common reasons for referring children to emergency pediatric tertiary centers. The first seizure does not suggest a specific neurological disorder; however, it can be a sign of increased irritability of the nervous system, which requires further diagnostic workup and follow-up (1). Seizure is a clinical manifestation of concomitant and abnormal neuronal stimulation in the cerebral cortex (2). This neuronal arousal is usually short-term (seconds to minutes) and self-limiting (3). Approximately 1.5 million children under the age of 6 experience seizures each year, including about 2.4 million children under the age of 2 years in developing countries (4). Seizures have a variety of treatable and untreatable but controllable etiologies. By identifying treatable causes, recurrence of seizures could be prevented, and by diagnosing controllable causes, intelligence, brain, and behavioral complications of seizures could be prevented. A seizure workup begins with a thorough history and neurological examination (5). To differentiate seizures from seizure mimickers as well as to determine the type of seizure, electroencephalography (EEG) is usually the preferred primary para-clinical method that has been recommended by the American Epilepsy Society (AES) as the standard tool for initial assessment of seizure (6). The electrical activity of the brain is recorded continuously during long-term monitoring (LTM) or by video EEG monitoring (7). Unfortunately, in many cases, electrical charges do not occur due to the absence of a seizure during EEG recording, but continuous long-term monitoring can record epileptic discharges (8). The most important use of LTM in clinical practice is identifying patients who are candidates for surgery (9). Other indications for LTM include children with refractory seizures to differentiate between seizures and non-epileptic events, children with recurrent or persistent seizures, differentiating the different types of seizures, and children with morbidities caused by refractory seizures (10-12). In pediatric practice, in addition to EEG as the standard method, imaging is also strongly recommended to functionally and anatomically evaluate the causes of seizures. In this regard, MRI is the modality of choice due to its high resolution and lack of radiation (13). MRI is significantly more sensitive than computed tomography (CT) for the evaluation of the hippocampus as one of the most common sites of seizure onset (13). However, a CT scan can be used as an MRI supplement to assess calcified brain lesions or, in emergency cases, to rule out intra-cerebral hemorrhage or cerebral hernia (14). Generally, the indication for emergency brain imaging is to detect focal neurological defects in the postictal phase (15). Non-emergency brain imaging in children with seizures is performed if there is a strong clinical suspicion of possible brain damage, including signs of cognitive or motor impairment, abnormal neurological examination, recent onset of focal seizures in children under one year of age, or the evidence of focal seizures in EEG (16). Magnetic resonance imaging is also used for predicting the prognosis and progression of neurological lesions in children with seizures (17). Abnormal findings on the brain MRI in children with refractory focal seizures have prognostic significance. It should be noted that any abnormal MR finding (including arachnoid cyst, diffuse atrophy, or ventricular asymmetry) should not be certainly considered as the cause of seizures. Thus, correlation of MRI findings with clinical evidence, neurological examination, and EEG are necessary (18). It has been shown that abnormal MRI findings in children with seizures are significantly associated with abnormal findings on EEG, patient age, family history of seizures, and abnormal neurological examination (19). In cases of drug-resistant and refractory seizures, the discovery of a resectable lesion in the same area as abnormal discharges in LTM on an MRI is extremely beneficial for both neurologists and neurosurgeons. This can potentially provide the patient with the opportunity to become seizure-free through the resection of the identified lesion. Although LTM and MRI are key technologies in the presurgical evaluation of patients with drug-resistant seizures, there is no study to evaluate the agreement between them in Iran and tertiary referral hospitals, which started epilepsy-specific MRI in 2017. Therefore, Further investigation is warranted to establish an agreement between EEG results and imaging in these children.
2. Objectives
The present study aimed to assess the agreement between abnormal findings in LTM and brain MRI as the standard imaging in children with focal epileptic discharges in LTM.
3. Methods
This research was carried out in compliance with the Helsinki Declaration and was approved by the Research Ethics Committee at the Tehran University of Medical Sciences (IR.TUMS.CHMC.REC.1397.025). This cross-sectional study was performed on 95 consecutive children who suffered from focal seizures or drug-resistant seizures with evidence of focal epileptic discharges in LTM and were referred to the Children's Medical Center in 2017. Informed consent was obtained from all parents. All children underwent brain MRI with epilepsy protocol afterward. Brain MRI was done for all patients with PHILIPS 1.5T MRI Machine and MRI protocol for epilepsy as a group of sequences (3DT1W,3D Flair, coronal and axial T2W, DWI, venous bold, and DTI). All MRIs were evaluated two times with one radiologist; the first time, she was blinded to the LTM findings, and the second time, she knew the zone of abnormal discharges in LTM. Those with a history of traumatic brain injury, acute infection, febrile illness, or evidence of chronic neurological disorders such as cerebral palsy, mental retardation, or progressive brain disorders were excluded from the study. Demographic information, including age and gender, time onset of seizures, type of seizure, family history of seizures, medical and surgical history, medication, and details of neurological examination, were all collected retrospectively using patients’ hospital records. In addition, abnormal EEG findings during the attacks (ictal EEG), EEG findings between attacks (interictal EEG), seizure focus location, brain MRI findings, and the LTM data were also collected by reviewing the files. The study aimed to determine the type of lesions detected in MRI and to assess the agreement between the location of these findings in MRI with LTM.
For statistical analysis, results were presented as mean ± standard deviation (SD) for quantitative variables and were summarized by frequency (percentage) for categorical variables. Continuous variables were compared using a t-test or Mann-Whitney test whenever the data did not appear to have normal distribution or when the assumption of equal variances was violated across the study groups. Categorical variables were, on the other hand, compared using the chi-square test. To assess the correlation between MRI findings and LTM, the Kappa agreement value was measured. P values ≤ 0.05 were considered statistically significant. For the statistical analysis, SPSS version 23.0 for Windows (IBM, Armonk, New York) was used.
4. Results
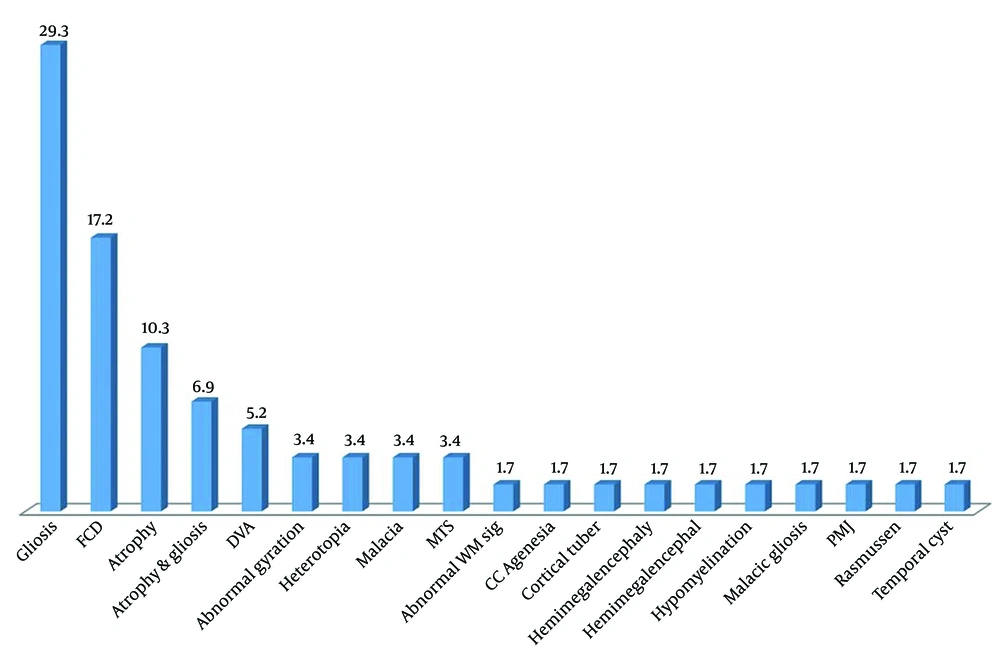
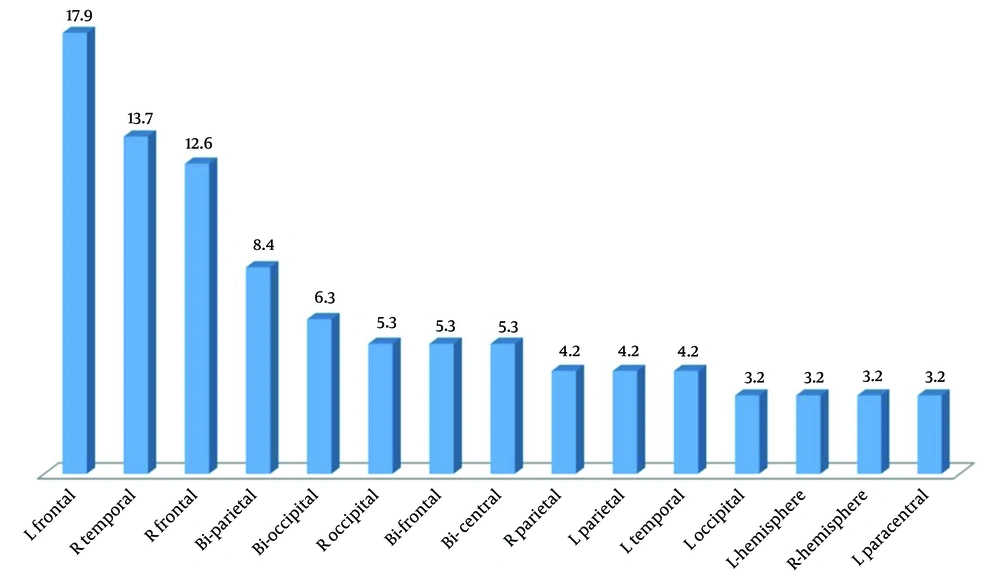
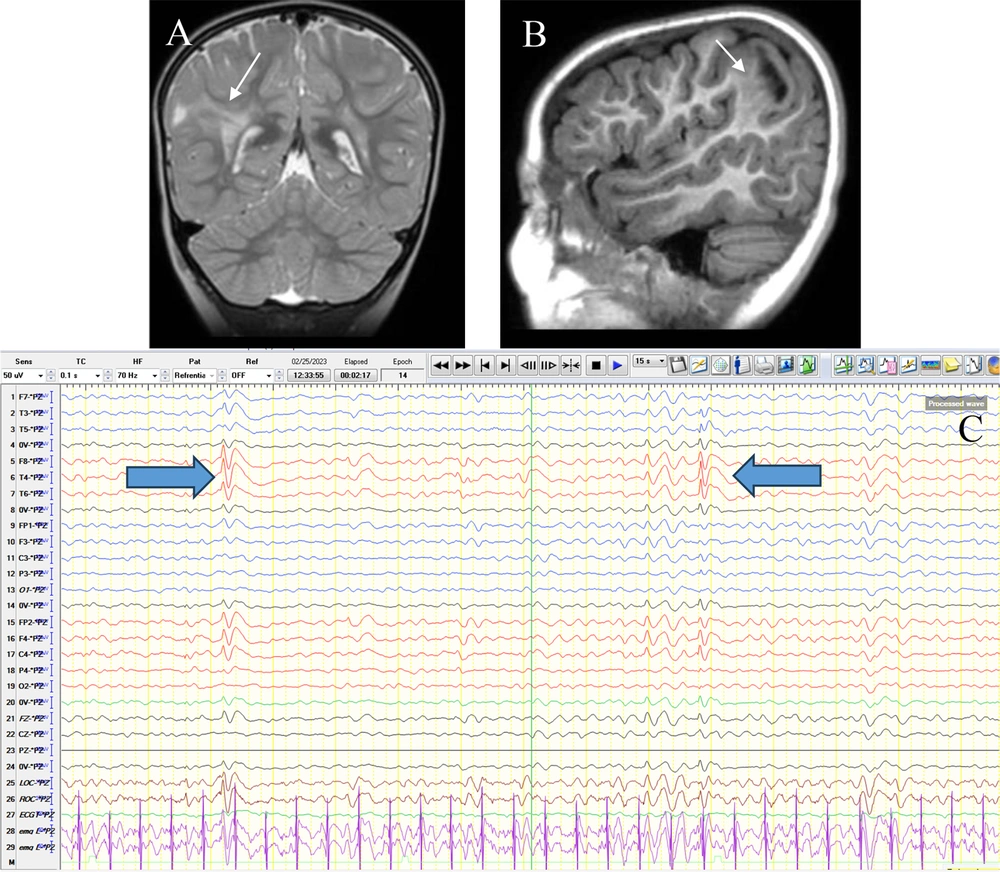
In total, 95 patients with abnormal LTM (53 boys and 42 girls) with an average age of 6.00 ± 3.64 years (ranging from 1 month to 17 years) were included in the study, among whom 59 children (62.1%) had abnormal MRI findings. Baseline characteristics in the two subgroups with and without MRI abnormalities are shown in Table 1. Abnormal neurological examination and prenatal problems were higher in the children with abnormal MRI findings in comparison with those with normal brain MRI (Table 1). As presented in Figure 1, the most common abnormal MRI finding was gliosis (29.3%), followed by focal cortical dysplasia (17.2%) and atrophy (10.3%). Figure 2 shows the most frequent findings in LTM, including left frontal involvement in 17.9%, right temporal involvement in 13.7%, and right frontal involvement in 12.6%. Figure 3 illustrates an instance of consistent abnormality in both LTM and MRI. Out of 95 patients with abnormal LTM, 59 had also abnormal MRI features. In this regard, concomitant right hemisphere involvement in both LTM and MRI was found in 39.0%, concomitant left hemisphere involvement in 28.8%, and involvement of both right and left hemispheres in 18.6% (Table 2). The diagnostic agreement between the MRI and LTM in discovering abnormal findings was found to be high (86.4%) with a kappa correlation coefficient equal to 0.79.
Brain MRI without gad in coronal T2W (A) and sagittal T1W (B) sequences show FCD type IIb with transmantle sign(thin arrow). This trace shows inter-ictal discharges (IEDs) as right-sided (predominantly parietal) polyphasic sharps (thick arrow) predominantly in a referential montage (Pz reference) (C).
| Characteristics | Normal MRI (n = 38) | Abnormal MRI (n = 57) | P-Value |
|---|---|---|---|
| Age, Mean ± SD (y) | 6.15 ± 3.07 | 6.10 ± 4.00 | 0.75 |
| Male/female ratio | 25/13 | 28/29 | 0.11 |
| Prenatal problems | 0 (0.0) | 24 (42.1) | <0.0001 |
| Abnormal neurological problems | 2 (5.3) | 16 (28.1) | 0.005 |
| Type of seizure | 0.05 | ||
| Focal | 21 (55.3) | 22 (39.6) | |
| Generalized | 11 (28.9) | 14 (24.6) | |
| Focal followed by generalized | 6 (15.8) | 21 (36.8) |
a Values are expressed as No. (%) unless otherwise indicated.
| Location of Abnormal LTM Discharges | Frequency | |||
|---|---|---|---|---|
| Right Lobe | Left Lobe | Bilateral | ||
| Location of abnormal MRI findings | ||||
| Right lobe | 23 (39) | 0 (0) | 3 (1.5) | 26 |
| Left lobe | 0 (1) | 17 (8.28) | 0 (1) | 17 |
| Bilateral | 2 (4.3) | 3 (1.5) | 11 (6.18) | 16 |
| Frequency | 25 | 20 | 14 | 59 |
a Values are expressed as No. (%).
5. Discussion
The Quality Standards Subcommittee of the American Academy of Neurology, along with the American Epilepsy Society, recommends EEG as the gold diagnostic standard for detecting afebrile childhood seizures. However, in children suffering from persistent postictal focal neurological deficits, MRI is now accepted as a preferred diagnostic modality, especially with the presence of abnormal neurologic signs. It remains unclear if MRI can guide treatment protocols (20, 21). Although EEG is the basis for clarifying the type of seizure or its onset zone, abnormal MRI findings can also show anatomical changes responsible for seizures. However, normal MR imaging does not mean that the underlying cause of neonatal seizures could be ruled out. In Sharma et al.'s study, only 26% of children with focal seizures had abnormalities found in their MRIs (22). In another study by Berg et al., only 12.7% of children with seizures had MRI-relevant lesions. MRI lesions have been reported in only 2.7% of children with seizures and without abnormal physical examination (23). In another study by King et al., lesions in MRI were found in only 14.0% of symptomatic children (24).
Magnetic resonance imaging could be routinely employed in children suffering from seizures, especially those with focal epilepsy or with the likelihood of secondary seizures. In the present study, about two-thirds of children with seizures and abnormal LTM showed significant MRI abnormalities. Also, we found high diagnostic agreement between MRI and LTM findings. It is likely that in children with new-onset seizures, the agreement between structural abnormalities identified on MRI and physiologic abnormalities identified on the EEG could assist in the discovery of the zone and the overall etiology of the seizure. When children experience refractory seizures and have two or more different lesions in their MRI or extensive abnormalities, invasive monitoring is necessary to locate the epileptogenic lesion. Our study revealed that the location of the lesion in MRI did not match the zone of abnormal discharges in LTM in 2 cases, indicating that any abnormal findings in MRI may not necessarily be the epileptogenic lesion. If a 1.5T MRI is non-lesional, a repeat scan is often conducted as a higher-field structural scan, typically a 3T (25). Doescher et al. showed a high rate of MRI abnormalities (32.6%) in children with normal EEG (26). They suggested that this low percentage of MRI abnormalities can impact disease treatment and prognosis. It should be noted that short-term and cross-sectional evaluation of such changes is inadequate, and follow-up of these patients is necessary to demonstrate the possible relevance of any of these abnormal MRI findings with disease course and prognosis (26).
It is important to acknowledge the limitations of this study. The primary limitations were related to the equipment and technology. Specifically, we utilized a 1.5T MRI instead of a higher-field 3T MRI, which would have produced clearer brain images (27). Additionally, we used a venous bold sequence instead of SWI and a PET scan instead of ASL. To improve future studies, we suggest evaluating the relationship between various clinical factors such as the type of seizure, treatment administered, time of onset, and the duration of the conflict. Furthermore, designing a study to assess patients with refractory or drug-resistant seizures and an MRI lesion before and after surgery would be of great value.
5.1. Conclusions
Two-thirds of patients with abnormal LTM findings had concurrent abnormal MRI features with high agreement values between the two modalities. Therefore, MRI and EEG can be very valuable in drug-resistant patients who may benefit from epilepsy surgery.