1. Background
Transient tachypnea of the newborn (TTN) is a common clinical condition characterized by transient rapid respiratory episodes, particularly observed in term and late-preterm infants. It typically occurs shortly after birth due to delayed clearance of fetal lung fluid. TTN usually resolves spontaneously, with supportive care being the primary approach to its management (1-3). However, emerging evidence suggests that TTN may be a risk factor for the development of childhood wheezing attacks or asthma (4-7).
The estimated incidence of TTN is 0.5% to 2.8% of all live births, with risk factors including cesarean delivery, male sex, lower gestational age, maternal history of asthma, and maternal diabetes (2, 8-11). Additionally, large cohort studies have shown that children diagnosed with TTN in the neonatal period experience more frequent wheezing attacks or asthma during school-age and adolescent years compared to those without respiratory problems during this period (4-7, 12, 13). It has been suggested that TTN could potentially serve as an indicator of compromised pulmonary function, implying an inherent predisposition to asthma in later childhood. However, asthma is a multifaceted disorder influenced by various factors. It has been postulated that these factors might contribute to both TTN and subsequent asthma development, or alternatively, that TTN itself may act as a risk factor for the later onset of asthma (2, 3).
The Impulse Oscillometry System (IOS) is a widely utilized method for lung function testing that offers diagnostic and monitoring capabilities for respiratory diseases. This non-invasive technique requires only passive cooperation from the patient and does not involve forced expiratory maneuvers. As a result, IOS has become a valuable tool for assessing lung function in children (14, 15).
2. Objectives
The aim of this study is to evaluate lung function in preschool children who were diagnosed with TTN in the neonatal period using the IOS device, to investigate the presence of respiratory morbidity in childhood and the factors influencing it.
3. Materials and Methods
3.1. Study Participants and Design
The study was conducted prospectively by the Department of Neonatology at Kocaeli University between 2014 and 2019, following approval from the ethics committee. The TTN group consisted of preschool children, aged between 3 and 7 years, who had previously been admitted to the neonatal intensive care unit with a diagnosis of TTN. These children were contacted through their registered phone numbers. Patients with congenital heart, pulmonary, neurological, or muscle diseases were excluded from the study. Perinatal history and clinical follow-up data were collected from hospital records. Information regarding doctor-diagnosed personal or family history of wheezing, asthma, and atopic dermatitis, as well as exposure to passive smoking (PS) at home and its duration, was also collected.
The age-matched healthy control group was selected from pediatric outpatient clinics based on criteria proposed by the American Thoracic Society. Exclusion criteria for the control group included a history of premature birth or low birth weight, previous supported ventilation therapy, being overweight, presence of heart, muscle, or neurological diseases, diagnosis of any respiratory disease, and exposure to PS (16).
Both the TTN group and the healthy controls were excluded if they had a respiratory tract infection within the two weeks preceding the study. The TTN group was asked to discontinue treatment with any inhaled medications (bronchodilators, steroids) at least one month before the study assessments were conducted. After physical examination, lung function tests were performed using an IOS device for both groups. In the TTN group, IOS testing was followed by blood sampling and skin-prick testing, as well as asthma or wheezing screening using the Modified Asthma Predictive Index (API) or the International Study of Asthma and Allergies in Childhood (ISAAC) (17, 18). Prior to the study, written informed consent was obtained from the parents of all participating children.
3.2. Transient Tachypnea of the Newborn Diagnosis
The diagnosis of TTN was established based on the following criteria: (1) a respiratory rate exceeding 60 breaths per minute within six hours after birth, accompanied by grunting sounds during breathing, flaring of the nostrils, and retractions; (2) tachypnea persisting for a minimum of 12 hours; (3) a chest radiograph indicating at least one of the following: Prominent central vascular markings, widened interlobar fissures or pleural fluid, symmetrical perihilar congestion, hyperaeration evidenced by flattening and depression of the diaphragmatic domes, or increased anteroposterior diameter, or both; and (4) exclusion of all other known respiratory disorders (such as meconium aspiration, respiratory distress syndrome, pneumonitis, congenital cardiac diseases) and non-respiratory disorders (such as hypocalcemia, persistent hypoglycemia, polycythemia) likely to cause tachypnea, based on radiological and laboratory findings (2, 19).
3.3. Impulse Oscillometry System
We used the Jaeger MasterScreen IOS system (Jaeger, Wurzburg, Germany) for our study. The procedure was explained to the children, and they were instructed to sit still, use a nose clip, and breathe quietly through a mouthpiece. At each test time point, three measurements were obtained, and the best measurement with regular breathing was selected for analysis and graphical representation (14, 15, 20).
The IOS device generates small pressure oscillations to assess the impedance (Z) of the respiratory system during regular breathing. Impedance consists of pulmonary resistance (R) and reactance (X). Resistance quantifies the energy required for the propagation of pressure waves through the airways, while reactance reflects the elastic recoil characteristics of lung tissue. Both R and X values are measured within the frequency range of 5 - 20 Hz. R5 and X5 serve as indicators of peripheral airway function, providing insights into the status of smaller airways. In contrast, R20 provides information about the functioning of central airways. The difference between R5 and R20 (R5 minus R20; R5-R20) serves as a sensitive indicator of the presence of peripheral airway obstruction. X5 encompasses the combined effects of lung elasticity and inertia, providing insights into the elastic recoil of the peripheral airways. Fres, known as resonant frequency, represents the point at which the lungs transition from passive distension to active stretching on the graph. The reactance area (AX) represents the cumulative area under the reactance curve between 5 Hz and Fres, encompassing all frequencies, and serves as a composite index for reactance. X5, Fres, and AX can be utilized as indicators to assess the extent of obstruction in the peripheral distal airways. The measurements for R and X can be reported in pascal (Pa) or cmH2O/L/s. X becomes zero at Fres, and the value of Fres is influenced by factors such as chest size and tissue properties (14, 15, 20, 21).
3.4. Asthma Screening
The modified API is a tool used to diagnose asthma in children under the age of 6 who have experienced four or more episodes of wheezing within one year. This diagnostic tool considers major risk factors, such as doctor-diagnosed asthma in the mother or father, doctor-diagnosed atopic dermatitis, and sensitivity to at least one aeroallergen, as well as minor risk factors, such as peripheral eosinophilia (> 4%), wheezing without colds, and food sensitivities. Non-asthmatic causes of wheezing are excluded from the diagnosis, and the presence of either one major or two minor criteria is required to confirm the diagnosis of asthma (17).
In children older than 6 years of age, the ISAAC questionnaire was used to assess the presence of respiratory symptoms, with or without rhinitis and eczema, within the past year. The questions included: "Has your child ever had wheezing or whistling in the chest at any time in the past?"; "Has your child had wheezing or whistling in the chest in the past 12 months?"; "Has your child ever had asthma?" These questions were further qualified by severity and frequency questions, such as: "How many attacks of wheezing has your child had in the past 12 months?"; "How often, on average, has your child’s sleep been disturbed due to wheezing?"; "In the past 12 months, has wheezing ever been severe enough to limit your child’s speech to only one or two words at a time between breaths?" (18).
The evaluation of reversibility and variability in the bronchodilator response, as well as treatment during follow-up, was planned for patients diagnosed with asthma.
3.5. Statistical Analysis
The data were analyzed using the IBM SPSS 25.0 (SPSS Inc., Chicago, IL, USA) software package. Descriptive statistics were reported as median/interquartile range (25th - 75th percentile) for continuous variables and as counts (percentages) for categorical variables. The normality of the data was assessed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Since the IOS values did not follow a normal distribution, comparisons between groups were conducted using the Mann-Whitney U test. A P-value of less than 0.05 was considered statistically significant, with a Type 1 (α) error level set at 0.05.
4. Results
4.1. The Characteristics of Participants
The study enrolled a total of 52 children diagnosed with TTN during the neonatal period into the TTN group, and 101 children were included in the control group. In the TTN group, there was a predominance of males (n = 33, 63.5%). No statistically significant differences were observed between the groups regarding anthropometric measurements. The mean ages of the TTN group and the control group were 60.4 ± 16.8 and 61.3 ± 15.5 months, respectively. The weight and height percentiles of children in both groups were within the normal range.
In the neonatal period, the median birth weight in the TTN group was 2695 (2207.5 - 3062.5) grams, and the median gestational age was 36.2 (35.3 - 37) weeks. Of these, 9.6% were large for gestational age (LGA, > 4000 grams). The majority of the children were delivered via cesarean section (88.5%). The rate of need for nasal continuous positive airway pressure (NCPAP) was 53.9%, and the duration of NCPAP therapy was 24 (18.5 - 30) hours. The median duration of total oxygen support was 24 hours.
In the TTN group, inhaler use was irregular for all but two patients, and a definitive diagnosis of asthma during the preschool period was not established. However, the rates of variable respiratory symptoms and ≥ 4 wheezing attacks in one year were 20.5% and 18%, respectively, resulting in a total of 38.5%, with 13.5% of the cases occurring in children older than 6 years of age. The rates of major risk factors associated with the modified API were as follows: Doctor-diagnosed parental/maternal asthma at 36.5%/5.8%, doctor-diagnosed atopic dermatitis at 4%, and aeroallergen sensitivity at 9.6%. Regarding minor risk factors, the rates were as follows: Wheezing without a cold at 32.7%, eosinophilia at 25%, and food allergy sensitivity at 15.4%. As most of these patients were under 6 years old, the modified API was applied to the majority of them (n = 35). Additionally, 46.2% of the group had been exposed to PS from birth (Table 1).
| TTN-Group, n = 52 | Mean ± SD/Median (25th - 75th p)/No. (%) |
|---|---|
| Median age, (mo) | 60.4 ± 16.8 |
| Male | 33 (63.5) |
| Median gestational age, (w) | 36.2 (35.3 - 37) |
| Median birth weight, (g) | 2695 (2207.5 - 3062.5) |
| Delivered via caesarean section | 46 (88.5) |
| Needed for nasal CPAP in the neonatal period | 28 (53.9) |
| History of doctor-diagnosed maternal asthma | 3(5.8) |
| History of doctor-diagnosed paternal asthma/atopy | 19 (36.5)/2(4) |
| Respiratory symptoms and ≥ 4 wheezing attacks within one year | 20 (38.5) |
| Having wheezing without a cold | 17 (32.7) |
| Doctor-diagnosed atopic dermatitis | 2 (4) |
| Food sensitivity | 8 (15.4) |
| Aeroallergen sensitivity | 5 (9.6) |
| Eosinophilia | 13 (25) |
| Allergic rhinitis | 7 (13.5) |
| History of doctor-diagnosed asthma | 2 (3,8) |
| PS expose from birth | 24 (46.2) |
Abbreviations: TTN, transient tachypnea of the newborn; PS, passive smoking; CPAP, continuous positive airway pressure.
4.2. Impulse Oscillometry System Results of the Participants
Significant differences were observed between the TTN group and the control group in the median IOS values of R5-R20, X10, and X15. Specifically, the median R5-R20 value was significantly higher in the TTN group, while the median X10 and X15 values were significantly lower compared to the control group (P < 0.05) (Table 2).
| IOS | TTN-Group (n = 52) | Control Group (n = 101) | P-Value | ||
|---|---|---|---|---|---|
| Median | 25th - 75th Percentiles | Median | 25th - 75th Percentiles | ||
| R5kPa/(L/s) | 0.87 | (0.75) - (1.08) | 0.86 | (0.67) - (0.97) | 0.102 |
| R10kPa/(L/s) | 0.72 | (0.64) - (0.88) | 0.71 | (0.61) - (0.82) | 0.345 |
| R15kPa/(L/s) | 0.66 | (0.60) - (0.86) | 0.70 | (0.58) - (0.79) | 0.810 |
| R20kPa/(L/s) | 0.61 | (0.52) - (0.81) | 0.65 | (0.55) - (0.76) | 0.868 |
| R5-R20kPa/(L/s) | 0.23 | (0.17) - (0.35) | 0.19 | (0.12) - (0.22) | 0.018 |
| X5 kPa/(L/s) | -0.23 | (-0.31) - (-0.19) | -0.24 | (-0.31) - (-0.17) | 0.873 |
| X10kPa/(L/s) | -0.15 | (-0.21) - (-0.10) | -0.12 | (-0.17) - (-0.06) | 0.010 |
| X15kPa/(L/s) | -0.10 | (-0.15) - (-0.05) | -0.07 | (-0.10) - (-0.02) | 0.033 |
| X20kPa/(L/s) | -0.01 | (-0.06) - (0.03) | 0.01 | (-0.03) - (0.04) | 0.060 |
| Resfreq 1/s | 20.67 | (17.34) - (23.39) | 19.41 | (17.48) - (21.86) | 0.138 |
| Z5kPa/(L/s) | 0.92 | (0.79) - (1.10) | 0.89 | (0.72) - (1.02) | 0.253 |
| AX (kPa/(L) | 1.50 | (0.84) - (1.95) | 1.53 | (0.86) - (1.95) | 0.879 |
Abbreviations: IOS, impulse oscillometric study; TTN, transient tachypnea of the newborn; R, resistance; X, reactance; resfreq, resonance frequency; AX, reactance area; Z, impedance.
In addition, participants in the TTN group were compared regarding several clinical factors that could potentially influence the IOS results.
4.3. Effects of Gestational Age on IOS Values of Transient Tachypnea of the Newborn Group
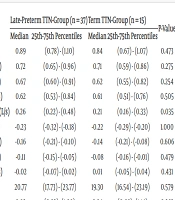
The TTN group was subdivided into late-preterm (n = 37, born at 340/7 - 366/7 weeks) and term (n = 15) groups based on gestational age at birth. The median R5-R20 and AX values of the IOS were significantly higher in the late-preterm subgroup compared to the term group (P < 0.05). No significant differences were observed between these subgroups in terms of other parameters (P > 0.05) (Table 3).
| IOS | Late-Preterm TTN-Group (n = 37) | Term TTN-Group (n = 15) | P-Value | ||
|---|---|---|---|---|---|
| Median | 25th-75th Percentiles | Median | 25th-75th Percentiles | ||
| R5kPa/(L/s) | 0.89 | (0.78) - (1.10) | 0.84 | (0.67) - (1.07) | 0.473 |
| R10kPa/(L/s) | 0.72 | (0.65) - (0.96) | 0.71 | (0.59) - (0.86) | 0.275 |
| R15kPa/(L/s) | 0.67 | (0.60) - (0.91) | 0.62 | (0.55) - (0.82) | 0.254 |
| R20kPa/(L/s) | 0.62 | (0.53) - (0.84) | 0.61 | (0.51) - (0.76) | 0.505 |
| R5-R20kPa/(L/s) | 0.26 | (0.22) - (0.48) | 0.21 | (0.16) - (0.33) | 0.035 |
| X5 kPa/(L/s) | -0.23 | (-0.32) - (-0.18) | -0.22 | (-0.29) - (-0.20) | 1.000 |
| X10kPa/(L/s) | -0.16 | (-0.21) - (-0.10) | -0.14 | (-0.21) - (-0.08) | 0.606 |
| X15kPa/(L/s) | -0.11 | (-0.15) - (-0.05) | -0.08 | (-0.16) - (-0.01) | 0.479 |
| X20kPa/(L/s) | -0.02 | (-0.07) - (0.02) | 0.01 | (-0.05) - (0.04) | 0.431 |
| Resfreq 1/s | 20.77 | (17.73) - (23.77) | 19.30 | (16.54) - (23.19) | 0.579 |
| Z5kPa/(L/s) | 0.92 | (0.82) - (1.09) | 0.84 | (0.68) - (1.14) | 0.363 |
| AX (kPa/L) | 1.24 | (0.65) - (1.54) | 0.80 | (0.55) - (1.34) | 0.049 |
Abbreviations: IOS, impulse oscillometric study; TTN, transient tachypnea of the newborn; R, resistance; X, reactance; resfreq, resonance frequency; AX, reactance area; Z, impedance.
4.4. Effects of Nasal Continuous Positive Airway Pressure on Impulse Oscillometry System Values of Transient Tachypnea of the Newborn Group
The TTN group was subcategorized based on whether they required NCPAP and then compared in terms of IOS results. The median AX value was significantly higher in infants who needed NCPAP (n = 28) (P = 0.018). However, there were no significant differences between these subgroups in terms of other IOS parameters (P > 0.05).
4.5. Effects of Maternal Asthma on Impulse Oscillometry System Values of Transient Tachypnea of the Newborn Group
Similarly, the TTN group was compared in terms of IOS results among children with and without a history of maternal asthma. Significant differences were found in the median values of R5, R10, R15, and R20. Children with a maternal history of asthma (n = 3) had significantly higher values compared to those without (n = 49) (P < 0.05).
4.6. Effects of Paternal Asthma/Atopy on Impulse Oscillometry System Values of Transient Tachypnea of the Newborn Group
There was no significant difference in IOS values when the TTN group was subcategorized based on the presence or absence of a history of paternal asthma/atopy (P > 0.05).
4.7. Asthma Diagnosis in Transient Tachypnea of the Newborn Group
According to the data, the rate of newly diagnosed asthma by a doctor in the TTN group was found to be 30.8% (n = 16) at the end of the study.
4.8. Effects of Passive Smoking Exposure on Impulse Oscillometry System Values of Transient Tachypnea of the Newborn Group
The TTN group was divided into two subgroups based on their exposure to PS: The PS group (n = 24) and the non-PS group (n = 28). The comparison between these subgroups revealed significant differences in the median IOS values of R5, R10, R15, Z5, X10, X15, and X20. Specifically, the PS group showed significantly higher median R5, R10, R15, and Z5 values and lower median X10, X15, and X20 values compared to the non-PS group (P < 0.05) (Table 4).
| IOS | PS TTN-Group (n = 24) | NonPS TTN-Group (n = 28) | P-Value | ||
|---|---|---|---|---|---|
| Median | 25th-75th Percentiles | Median | 25th-75th Percentiles | ||
| R5kPa/(L/s) | 0.93 | (0.81) - (1.21) | 0.82 | (0.72) - (1.02) | 0.019 |
| R10kPa/(L/s) | 0.79 | (0.71) - (1.01) | 0.67 | (0.62) - (0.85) | 0.022 |
| R15kPa/(L/s) | 0.71 | (0.62) - (0.97) | 0.63 | (0.55) - (0.83) | 0.043 |
| R20kPa/(L/s) | 0.65 | (0.56) - (0.89) | 0.59 | (0.49) - (0.76) | 0.110 |
| R5-R20kPa/(L/s) | 0.28 | (0.25) - (0.39) | 0.23 | (0.21) - (0.26) | 0.088 |
| X5 kPa/(L/s) | -0.22 | (-0.32) - (-0.19) | -0.23 | (-0.31) - (-0.18) | 1.000 |
| X10kPa/(L/s) | -0.18 | (-0.23) - (-0.13) | -0.14 | (-0.19) - (-0.07) | 0.043 |
| X15kPa/(L/s) | -0.12 | (-0.16) - (-0.09) | -0.06 | (-0.15) - (-0.01) | 0.015 |
| X20kPa/(L/s) | -0.04 | (-0.07) - (0.01) | 0.01 | (-0.05) - (0.06) | 0.040 |
| Resfreq 1/s | 21.94 | (19.78) - (23.39) | 19.13 | (15.60) - (23.43) | 0.091 |
| Z5kPa/(L/s) | 0.97 | (0.86) - (1.21) | 0.84 | (0.70) - (1.06) | 0.017 |
| AX (kPa/(L) | 0.89 | (0.65) - (1.64) | 0.87 | (0.63) - (1.62) | 0.319 |
Abbreviations: IOS, impulse oscillometric study; PS, passive smoking; TTN, transient tachypnea of the newborn; R, resistance; X, reactance; resfreq, resonance frequency; AX, reactance area; Z, impedance.
5. Discussion
Although TTN is generally considered a benign and self-limiting condition in newborns, recent data suggesting a link between TTN and wheezing or asthma raises questions about whether TTN may be a precursor to childhood respiratory disease (2, 7). Our study showed that preschool children with a history of TTN may have peripheral airway obstruction, which is a characteristic finding of asthma. This was evident through significantly increased R5-R20 values and more negative X10 and X15 IOS values when compared with age-matched healthy controls. Additionally, we observed higher R values and AX values in subgroups with a history of late-preterm birth, noninvasive respiratory support, and maternal asthma. However, the effect of PS exposure on our preschool children's IOS values seemed more pronounced than the effects of known risk factors for TTN.
Because IOS is effort-independent and requires minimal patient cooperation, it is a valuable tool for assessing lung function in preschool children. One of the main applications of IOS in children is the assessment of patients with asthma. Typically, asthma patients with increased peripheral airway resistance exhibit elevated R5, more negative X5, and increased Fres values (14, 15, 22, 23). R5, R5-R20, and AX are widely recognized as the most sensitive IOS parameters for detecting peripheral airway obstruction, assessing the severity of asthma and exacerbations, and early identification of lung function abnormalities (23, 24). In our study, increased R5-R20 was a significant finding in preschool children diagnosed with TTN compared to healthy controls, indicating possible peripheral airway obstruction, in addition to the negative X values.
In a recent study, Klinger et al. reported that infants with TTN also had increased R and decreased reactance (X) compared to healthy term controls using the forced oscillation technique on the first day after birth (25). Although the aim of their study was to demonstrate the feasibility of FOT in neonates and its ability to distinguish normal control infants from those with TTN, it also showed the impact of TTN on lung function, particularly on oscillometric parameters R and X.
Cesarean section is recognized as a risk factor for TTN due to the absence of the natural surge of catecholamine that occurs during a vaginal delivery. This surge triggers a β-adrenergic receptor-mediated response, leading to fluid absorption in distal airways through Na pump activity (3, 26). Faxelius et al. found a significant correlation between catecholamine concentrations and lung compliance two hours after birth (26). Another study by Lee et al. assessed total body plethysmography and functional residual capacity using argon dilution in newborns, comparing those delivered vaginally to those born via elective cesarean section at 4-6 hours and 24 hours after birth. They observed a delay of up to 24 hours in achieving final lung volumes in infants born without exposure to labor or passage through the birth canal, which could potentially contribute to increased respiratory morbidity associated with elective cesarean section deliveries (27).
Furthermore, Owens et al. evaluated whether reduced lung function in early infancy is predictive of persistent asthma in young adults. They assessed lung function at various intervals: One, 6, and 12 months (using maximum expiratory flow at functional residual capacity, V'max), and at 6, 11, 18, and 24 years of age (using spirometry) in an unselected cohort of full-term births. Their findings demonstrated that neonatal impairment in lung function is a risk factor for persistent asthma throughout life (28). Similarly, our study also revealed significant differences in the IOS values of X10 and X15, which indicate compromised lung capacitance and elasticity, between preschool children under 7 years old diagnosed with TTN during the neonatal period and age-matched healthy controls.
Late-preterm infants have been reported to be associated with a greater incidence of cesarean delivery and TTN, frequently necessitating respiratory interventions such as mechanical ventilation and oxygen support. In a recent study, Gustafson et al. identified that exposure to any form of respiratory support is significantly associated with recurrent wheezing in late-preterm infants during the first three years of life (29). Our study also supported this finding, showing a significant increase in AX value, indicative of peripheral obstruction in IOS, among preschool children predominantly born late-preterm and requiring NCPAP, compared to those who did not require NCPAP. Since alveolar development continues until 8 - 10 years of age, postnatal exposure to any type of respiratory support has been considered to increase the risk of respiratory symptoms due to its potential inflammatory effects on lung tissue (29, 30).
Birth during the 340/7–366/7 gestational period disrupts critical phases of rapid in-utero respiratory growth, affecting the maturation of acinar structures and overall lung development. These disruptions can lead to alterations in pulmonary mechanics during infancy, including a compliant chest wall, reduced expiratory airflow, and increased airway resistance (31). Thunqvist et al., in a large-scale cohort study, conducted spirometry on children born moderate-to-late preterm (MLP) at 8 years of age and re-evaluated them at 16 years using both spirometry and oscillometry. The study found lower values of forced expiratory volume in one second (FEV1) and higher R5, R5-R20, and AX values, indicating airway obstruction in children born MLP compared to those born at term (13). Similarly, in our study using spirometry, we observed increased R5-R20 and AX values in children born late-preterm compared to those born at term. In contrast, Dantas et al. reported that lung parameters in school-aged children born MLP were similar to those born at term, suggesting continued pulmonary plasticity (32). However, a recent systematic review and meta-analysis found that MLP birth is associated with reduced expiratory airflow across childhood, adolescence, and adulthood. While the degree of airflow obstruction was modest, as indicated by differences in z-scores for FEV1, FEV1/FVC, and FEF25-75%, MLP-born individuals exhibited worse expiratory flows compared to term-born controls (31).
In a study conducted in 2007, Liem et al. identified several independent risk factors for the development of TTN, including birth weight ≥ 4500 g, maternal asthma, male sex, urban location, and cesarean section. Their findings also suggested that TTN could potentially serve as the initial presentation of asthma in preschool children with a maternal history of asthma (5). Later literature, based on surveys, has indicated that prematurity, family history of asthma, and maternal tobacco use are statistically significant predictors for the development of bronchial hyperactivity and asthma in childhood (29, 33-35). In another study, Mendola et al. showed that adverse neonatal outcomes, such as respiratory complications and NICU admission, increased with maternal asthma in term births (35, 36). Noting the link between β-adrenergic response and activation of Na transport, they suggested that one possible genetic predisposition was β-adrenergic hypo-responsiveness in those infants and mothers (11). In a cohort of 73 children where at least one parent was atopic, a reduction in neonatal VmaxFRC was demonstrated in children with a polymorphism in some allele of the β-adrenergic receptor gene (37). Additionally, a recent study reported that maternal asthma is linked to lower lung function in male babies, potentially affecting their future lung health and increasing the risk of wheezing and asthma (38). Despite the limited sample size, we also demonstrated a significant increase in distal airway resistance, reflected in all R values of IOS, among preschool children with a maternal history of asthma compared to controls. However, we found no significant difference in IOS values in the TTN group when subcategorized by paternal asthma/atopy history.
While genetic factors are believed to play a role in the occurrence of TTN and the subsequent development of asthma, environmental factors like exposure to PS can also exert a significant influence. A study conducted by Lajunen et al. aimed to investigate the impact of environmental tobacco smoke exposure on lung function in preschool children with asthma. They observed a significant association between cotinine levels and increased baseline R5 and decreased baseline X5 values. These IOS findings were notable for suggesting small peripheral airway dysfunction, resembling chronic obstructive pulmonary disease (39). Similarly, our study revealed significant differences in preschool children exposed to PS compared to those in the Non-PS group. Specifically, the PS group exhibited higher resistance and lower reactance in peripheral airways. The impact of PS exposure on IOS values in our study group was particularly pronounced.
We have previously shown that maternal smoking during the prenatal period significantly increased peripheral airway resistance, as measured by IOS, in preschool children born late-preterm (40). Bisgaard et al., in a prospective birth cohort study of 411 newborns, determined that neonates of mothers who smoked during the third trimester had a 7% loss in FEV at 0.5 seconds (41). It has also been noted that the interaction between intrauterine smoke exposure and transferase gene expression is linked to reduced functional residual capacity and hyperresponsiveness in exposed infants (42). Hence, it is plausible to suggest that airway remodeling may occur within the unique epigenetic environment of each individual child (39, 43).
Our study is important for evaluating the effects of a TTN diagnosis in the neonatal period on lung function using IOS in preschool-age children. However, it has certain limitations. The TTN group could have included more participants, especially those with a maternal history of doctor-diagnosed asthma. A larger sample size is needed to reach a definitive conclusion. This limitation may be due to difficulties in contacting children by phone and scheduling hospital visits for follow-ups over subsequent years. Factors like logistical challenges, geographical dispersion, and the complexity of obtaining detailed medical histories may have also contributed to the smaller sample size in the doctor-diagnosed maternal asthma subgroup. Additionally, some families may have been hesitant to commit to long-term research involvement.
Another limitation of our study is that parental reports were used to assess PS exposure, and we did not utilize biomarkers like urine cotinine levels to validate the exposure. Furthermore, we did not precisely quantify the duration of PS exposure or the number of smokers in the household. However, in a study investigating the effects of maternal smoking, a random sample from the cohort was used, and it was found that there was good agreement with serum cotinine levels (44). Additionally, the use of questionnaires to determine smoking status has proven to be a valuable tool in epidemiological research for assessing the adverse effects of PS. Even so, differences between lung function parameters in children exposed to PS and those who are not could provide a strategy for implementing control measures and informing smoking parents of the risks.
In conclusion, this study suggests that preschool children diagnosed with TTN during the neonatal period may exhibit peripheral airway obstruction, a characteristic of asthma, compared to age-matched healthy controls. The development of asthma is clearly a highly complex and multifactorial process. Identifying risk factors and understanding the mechanisms that lead to recurrent wheezing are crucial for pinpointing targets for primary prevention as well as future interventions and treatments. Being born late preterm, the effects of nasal mechanical ventilation, exposure to cigarette smoke, and a maternal history of asthma appear to be potential factors that could alter lung function in preschool children diagnosed with TTN during the neonatal period.
Families should be made aware of the potential long-term consequences of preterm birth and the damaging effects of cigarette smoke on lung function. Additionally, monitoring lung function, providing long-term medical care, and avoiding exposure to cigarette smoke should be emphasized throughout childhood and potentially into adulthood for patients diagnosed with TTN during the neonatal period. While our findings offer valuable insights, larger-scale studies are necessary to validate these results.