1. Background
Cystic abdominal lesions are relatively common in children and encompass a broad spectrum of conditions that can arise from almost any structure within the abdominal cavity. Although clinical presentation, along with factors such as age, symptoms, and laboratory findings, aids in diagnosis, these lesions can resemble others with similar presentations. Thus, imaging plays an essential role in the diagnostic process (1). Identifying the originating organ is the initial step in diagnosing an abdominal mass. However, this task becomes more challenging when the lesion does not originate from the parenchyma of a solid organ or in cases of parenchymatous lesions, particularly with large cysts, complicating the differential diagnosis process.
Ultrasonography (US) is widely recognized and utilized as a safe imaging method and is now the first-line technique for evaluating abdominal emergencies in newborns and children. Radiologists can obtain high-resolution images without exposing patients to ionizing radiation, potentially providing a specific diagnosis or narrowing the differential diagnoses (2).
While other imaging modalities, like computed tomography (CT) scans, can offer additional information (3), they suffer from inherent weaknesses in spatial resolution, failing to capture fine details of cysts, such as fine septa, wall layers, and fluid debris levels, which are crucial for determining the nature of the cysts. Moreover, concerns over radiation exposure from diagnostic CT scans often dissuade radiologists from using this modality. MRI, although useful, comes with its own set of limitations, including cost, limited availability, and, in some cases, the need to sedate the child, rendering it a less accessible option in many facilities (4).
Advancements in US technology, particularly the use of high-frequency linear probes for children, have solidified the role of ultrasound (US) as an indispensable tool in pediatric imaging. This is especially true for cystic masses, as fluid-filled structures provide excellent acoustic windows for evaluating internal contents and adjacent structures in detail (5). Assessing the US findings of these lesions and their diagnostic accuracy and specificity can significantly aid patient management. This approach enables radiologists and physicians to tailor treatment based on specific US features and determine the necessity for further imaging evaluations.
2. Objectives
This study aims to describe the characteristic US features of intra-abdominal cystic lesions not originating from the liver, spleen, or kidneys in children and to assess their diagnostic value in providing definitive diagnoses.
3. Methods
3.1. Study Settings and Population
This prospective cross-sectional study employed non-probability purposive sampling to include patients with intra-abdominal cystic lesions referred to two tertiary referral centers in Mashhad (Akbar and Dr. Sheikh Children's Hospital) over three years (2019 - 2022). The study encompassed children under 19 years old presenting with any abdominal cystic lesion on initial US examination. Exclusion criteria were patients with intra-parenchymal cysts in the liver, spleen, or kidneys, those with a history of surgery or interventional procedures affecting the US appearance of cysts, and those lost to follow-up. Ultimately, 104 children were evaluated, with 38 patients excluded from the study.
3.2. Ethical Considerations
Informed consent was obtained from the patient's parents or legal guardians prior to their inclusion in the study. The Research Ethics Committee of Mashhad University of Medical Sciences granted approval for this study under the code IR.MUMS.MEDICAL.REC.1400.617.
3.3. Data Collection
A single radiologist with 12 years of pediatric radiology experience conducted all sonographic examinations prospectively. Scans were performed using a real-time sonographic unit (Voluson E6, Samsung Model WS80, or Esoate Class C) equipped with 5-MHz convex transducers and 10-12-MHz linear transducers, utilizing standard settings. Besides routine abdominopelvic cavity scanning, particular attention was devoted to the US characteristics of cystic masses. Participants were followed up until a final diagnosis was reached. Follow-up US examinations were conducted for some patients to observe any changes in appearance or complications. Patients with duplication cysts, complicated, and symptomatic cysts underwent surgery, while others were managed clinically. Final diagnoses were based on histopathological reports, surgical findings, or follow-up imaging and compared with the initial US features.
3.4. Statistical Analyses and Sample Size
Data were analyzed using SPSS software, version 16.5. Descriptive methods such as frequency distribution, percentage, mean indices, standard deviations, and tables described the US features in patients. The agreement between US findings and final diagnoses after follow-up was assessed using the Kappa coefficient test, with an 80% power and 95% confidence level, aiming for a relative 10% accuracy. The sample size, determined based on a kappa coefficient of 0.5 for the null hypothesis and 0.8 for the alternative hypothesis, was initially set at 80 patients but was increased to 100 cases to account for a potential dropout rate of 10 - 20%. Sensitivity and specificity were compared and analyzed to evaluate the diagnostic accuracy of the US.
4. Results
After a thorough examination of the clinical and surgical histories and conducting US examinations, 142 children meeting the inclusion criteria were initially enrolled in our study. Throughout the study period, 25 patients were lost to follow-up, and 13 patients declined further imaging or surgical evaluations. Consequently, our study ultimately included 104 patients with intra-abdominal cystic lesions. The average age of the participants was 3.22 ± 4.3 years, ranging from 2 days to 19 years (Table 1). Distribution of the cystic lesions showed 52 cases (50%) located on the right side of the abdominal cavity, 25 cases (24%) on the left side, 13 cases (12.5%) in the hypogastric region, 11 cases (11.6%) in the epigastric region, and 3 cases (2.9%) centrally located.
| Parameters and Subgroups | Values a |
|---|---|
| Sex | |
| Male | 41 (39.4) |
| Female | 63 (60.5) |
| Age, y | |
| 0 - 4 | 58 (55.7) |
| 4 - 8 | 20 (19.2) |
| 8 - 12 | 17 (16.3) |
| 12 - 19 | 9 (8.6) |
a Values are expressed as No. (%).
The most frequent diagnoses identified by the US were perinatal ovarian torsion (22.1%, n = 23), intestinal duplication cysts (18.3%, n = 19), and ovarian follicular cysts (17.3%, n = 18). The prevalence and US characteristics of different intra-abdominal cyst types in our study are detailed in Table 2. Notable US features included the presence of a multilayered wall in perinatal twisted ovarian cysts (95.7%, n = 22) and duplication cysts (89.5%, n = 17), and the cyst-within-a-cyst sign observed in follicular cysts (72.2%, n = 13), as illustrated in Figure 1.
| Causes of Cyst | Number | Age, y | Female | Diameter, mm | Multilayered Wall | Debris | Septa | Calcification | Solid Content or Adjacent Solid Tissue | Cyst Within Cyst |
|---|---|---|---|---|---|---|---|---|---|---|
| Twisted Ovarian cyst | 23 | 0.03 | 23 (100) | 50.6 | 22 (96) | 21 (91) | 6 (26) | 1 (4) | 1 (4) (solid content) | - |
| Follicular cyst | 18 | 0.1 | 18 (100) | 43 | 3 (17) | - | 4 (22) | - | 5 (28) (adjacent solid tissue) | 13 (72) |
| Duplication cyst | 19 | 2.09 | 8 (42) | 39.4 | 17 (89) | 10 (53) | - | - | 1 (5) (solid content) | - |
| Pancreatic pseudocyst | 11 | 7.8 | 5 (45) | 36 | - | 9 (82) | 3 (27) | - | 1 (%) (solid content) | - |
| Mesenteric cyst | 8 | 4 | 3 (37) | 41 | - | 4 (50) | 7 (87) | - | 1 (solid content) | - |
| intraabdominal Sacrococcygeal teratoma | 6 | 3.33 | 4 (60) | 33.4 | - | 2 (33) | 6 (100) | 4 (67) | 6 (100) (solid content) | - |
| Sclerosing encapsulating | 3 | 12 | 0 (0) | 56.8 | - | - | 3 (100) | - | 1 (33) (solid content) | - |
| Urachal cyst | 2 | 4.5 | 1 (50) | 22.6 | - | 1 (50) | 2 (100) | - | - | - |
| Meconium cyst | 1 | 0.4 | 0 (0) | 28 | - | - | - | 1 (100) | - | - |
| Mesenteric hydatid cyst | 1 | 9 | 0 (0) | 53.8 | 1 (100) | - | - | - | - | - |
| Omphalomesentric cyst | 1 | 2.1 | 1 (100) | 34 | 1 (100) | - | - | 1 (100) | - | - |
| Organized collection | 2 | 7.5 | 0 (0) | 47.4 | - | - | 2 (100) | - | 2 (100) | - |
a Values are expressed as No. (%).
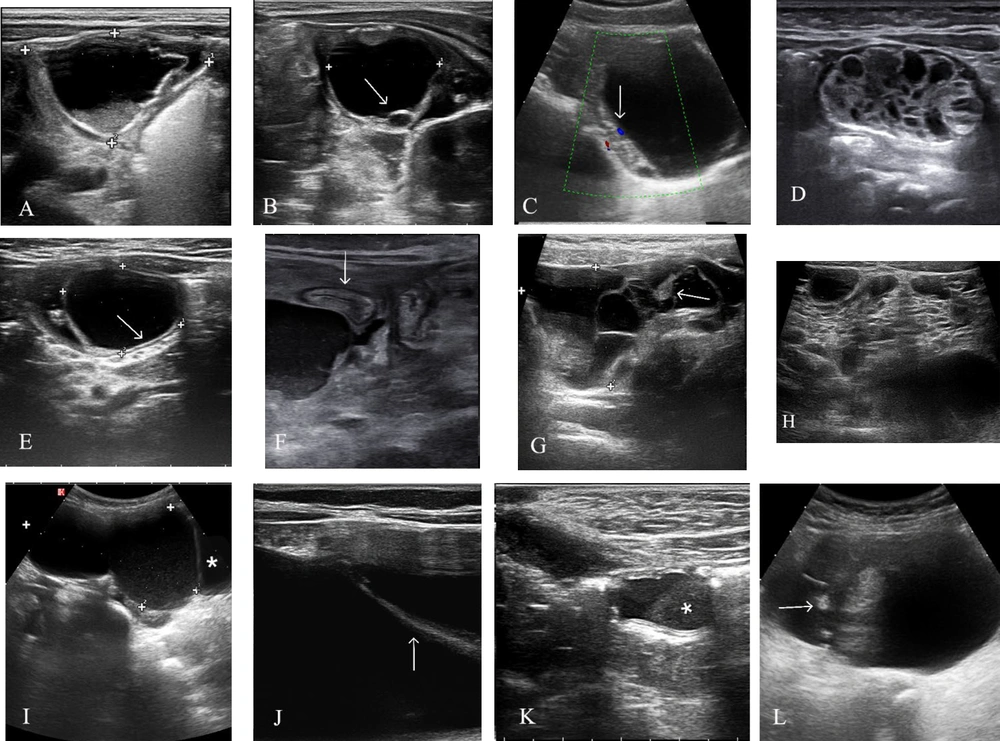
Ultrasonographic views of intra-abdominal cysts. A: A perinatal twisted ovarian cyst in a 20-day-old asymptomatic neonate, previously identified as a complex pelvic cyst on antenatal ultrasound. Pelvic ultrasound examination using a 12-MHz linear transducer displayed a cyst with a multilayered echogenic wall and a debris-fluid level (calipers). B: A pelvic ultrasound of a 12-year-old girl experiencing occasional abdominal pain revealed a large ovarian cyst containing a 'daughter cyst' (arrow), indicative of an ovarian follicular cyst. The cyst resolved six weeks later, as observed in a follow-up examination. C: An ultrasound of a 9-year-old child with acute abdominal pain showed a follicular cyst. The color Doppler examination revealed minimal vascularity in the adjacent solid tissue (arrow pointing to ovarian parenchyma). Surgery confirmed the right ovary was twisted about three times along its pedicle. D: An abdominal ultrasound (using a linear transducer) of a nine-day-old neonate with a prenatal diagnosis of an abdominal cyst revealed a complex multicystic mass with a small amount of free fluid in the right lower quadrant. Surgery found a quadruple-twisted ovary containing multiple hemorrhagic cysts. E: An ultrasound of a 2-year-old asymptomatic infant displayed an anechoic cystic lesion with a characteristic double wall sign (arrow), consistent with an enteric duplication cyst. No clear communication with the gastrointestinal tract lumen was observed on ultrasound. F: An ultrasound examination (using a 12-MHz linear transducer) of a 10-year-old boy with nonspecific abdominal pain and vomiting for the past two weeks showed a large retro gastric cystic structure containing mobile internal debris and a minimal amount of retroperitoneal fluid. The cyst, drained surgically, contained pancreatic enzymes, confirming a pancreatic pseudocyst. The arrow points to the stomach. G: A lower abdominal ultrasound revealed a multicystic peritoneal lesion (calipers) with an echogenic clot (arrow) and low-level debris encasing a small bowel loop. Pathological examination post-surgery confirmed a hemorrhagic peritoneal mesothelial cyst. H: A transverse ultrasound image of a four-year-old child illustrated a multiloculated cystic lesion filled with multiple thin septa, diagnosed as a multilocular chylolymphatic mesenteric cyst with various-sized cystic spaces on gross pathology (lymphatic malformation). I: A longitudinal ultrasound image using a 5-MHz convex transducer in a six-year-old patient with urinary incontinence revealed a large midline bilobed cyst with low-level echoes situated between the bladder and umbilicus. A thin fibrous tissue at its upper portion linked the cyst to the umbilicus, identifying it as a urachal cyst. The star (*) denotes the bladder. J: An axial ultrasound image of sclerosing encapsulating peritonitis in a 12-year-old boy undergoing long-term peritoneal dialysis displayed loculated fluid confined by a thickened peritoneum (arrow). K: An axial ultrasound image using a 12-MHz linear transducer depicted a wandering cystic lesion with a fluid-debris level (*) in the hypogastric region, distinguishable from urachal cysts by its off-midline position. No direct connection to the bladder or ileal loops was observed. L: A longitudinal ultrasound image of type IV cystic sacrococcygeal teratoma showed a cystic lesion containing hypoechoic and echogenic solid components along with calcifications (arrow).
Ultrasound exhibited a sensitivity of 82.61% (95% CI = 0.6 - 0.94) and specificity of 100% (95% CI = 0.94 - 1) for diagnosing perinatal ovarian torsion. It also showed 89.47% sensitivity (95% CI = 0.65 - 0.98) and 94.11% specificity (95% CI = 0.86 - 0.97) for identifying duplication cysts. In diagnosing pancreatic pseudocysts, ultrasound achieved 100% sensitivity (95% CI = 0.67 - 1) and 98.92% specificity (95% CI = 0.93 - 0.99). Mesenteric cysts were diagnosed preoperatively with ultrasound, showing 66.66% sensitivity (95% CI = 0.35 - 0.88) and 98.91% specificity (95% CI = 0.93 - 0.99).
When comparing US diagnoses with final diagnoses, US findings were in agreement with the final diagnoses in 84 (80.8%) patients. The US diagnosis included the final diagnosis in the differential for 8 (7.7%) patients. However, US findings were not in agreement with the final diagnosis in 12 (11.5%) patients. Detailed assessments for each lesion type are summarized in Table 3.
| Final Diagnosis | Age, y | Number of Females | Total Number of Patients | Follow-Up by Ultrasound | CT Scan | Surgery | MRI |
|---|---|---|---|---|---|---|---|
| Twisted ovarian cyst | 0.03 | 23 (100) | 23 | - | - | 23 (100) | 3 (13) |
| Follicular cyst | 0.1 | 18 (100) | 18 | 18 (100) | - | 3 (16) | - |
| Duplication cyst | 2.09 | 8 (42) | 19 | - | 8 (42) | 19 (100) | - |
| Pancreatic pseudocyst | 7.8 | 5 (45) | 11 | 8 (72) | 9 (81) | 3 (27) | - |
| Mesenteric cyst | 4 | 3 (37) | 8 | 5 (62) | 5 (62) | 4 (50) | 3 (37) |
| Intraabdominal sacrococcygeal teratoma | 3.33 | 4 (60) | 6 | - | 2 (33) | 6 (100) | 2 (33) |
| Sclerosing encapsulating | 12 | 0 (0) | 3 | 1 | 3 | 2 | 1 |
| Urachal cyst | 4.5 | 1 (50) | 2 | - | - | 2 | - |
| Meconium cyst | 0.4 | 0 (0) | 1 | - | - | 1 | - |
| Mesenteric hydatid cyst | 9 | 0 (0) | 1 | - | - | 1 | - |
| Omphalomesentric cyst | 2.1 | 1 (100) | 1 | - | 1 | 1 | - |
| Organized collection | 7.5 | 0 (0) | 2 | - | 2 | 2 | - |
Abbreviations: CT scan, computed tomography scan; MRI, magnetic resonance imaging.
a Values are expressed as No. (%).
5. Discussion
Accurate differentiation of lesions is crucial for the proper management of cystic abdominal masses. In this regard, imaging tools that emit low or no radiation are preferred for diagnosis. Ultrasound is one such imaging modality. While the imaging characteristics of various cystic abdominal masses, particularly intra-parenchymal cysts of the liver and kidney, have been well documented in the literature, the efficacy of US in differentiating these masses still needs further establishment. Specifically, there have been limited studies on the diagnostic accuracy of US for intra-abdominal cysts not associated with the liver, kidneys, or spleen. This gap in research is partly due to the relative rarity of these lesions and their varied nature, highlighting the need for additional studies.
In our study, the US diagnosis agreed with the final diagnosis in 80.8% of pediatric patients presenting with intra-abdominal cystic masses. These definitive diagnoses varied, including follicular ovarian cysts and cysts stemming from the gastrointestinal tract, such as duplication and pancreatic pseudocysts. Notably, the most frequently observed cystic mass was the perinatal twisted ovarian cyst, which was clearly identifiable on US imaging. Cystic ovarian masses, comprising follicular ovarian cysts and perinatal torsed ovarian cysts, were observed. Despite these lesions originating from the ovarian parenchyma, diagnosing infantile ovaries via US and categorizing them as parenchymal cysts can be challenging. Previous studies have identified many reported lesions as significant mimics of other abdominal cysts, such as intestinal duplication cysts or mesenteric cysts (6).
Literature indicates that ovarian cysts are the most prevalent congenital cysts in female fetuses. A retrospective analysis by Monnery-Noche et al. found that 56% of these perinatal cysts were twisted at the time of surgery (7). Sonographically, cystic ovarian masses are often indicative of perinatal ovarian torsion, frequently characterized by multilayered walls on US (8). In this study, the characteristic multilayered walls were observed in 96% of cases, with US demonstrating 82.6% sensitivity and 100% specificity in distinguishing perinatal twisted ovarian cysts from other intra-abdominal cysts. Perinatal ovarian torsions are often diagnosed late, presenting as cystic masses, whereas torsions at other ages are symptomatic, typically showing as an enlarged ovary with peripheral follicles. A retrospective study by Kim et al. on neonates with ovarian torsion found complex cysts with intracystic debris, the double-wall sign, fluid-fluid levels, and multiple septations in all patients (9). While some research indicates that cystic masses appear in up to 90% of cases with perinatal twisted ovarian cysts (10), there have also been instances of ovarian torsions occurring without any underlying pathology (11).
A 2015 meta-analysis indicated that B-mode US achieves an overall pooled sensitivity of 55% and a specificity of 87% in diagnosing ovarian torsion among pediatric patients (12). This suggests that B-mode US successfully identifies only 55% of ovarian torsion cases, though there is variability in the literature, with some studies suggesting that the pelvic US is 80% sensitive and 95% specific in diagnosing ovarian torsion (13). Our findings, however, demonstrate that when a cystic abdominal mass is present, US is highly specific in identifying cystic masses linked to ovarian torsion. A limitation of our study is the exclusive inclusion of cases with cystic abnormalities; thus, our results should be cautiously interpreted, as our sample does not encompass all instances of ovarian torsion (Figure 1A - D).
Another relatively prevalent abdominal cyst deriving from ovarian tissue is the ovarian follicular cyst, mostly observed in prepubertal children and adolescents due to follicles failing to involute under hormonal stimulation (14). A simple ovarian cyst is typically characterized as round to oval, featuring smooth, thin walls without any solid components or septations (15). In our research, a normal ovary was seen as solid tissue surrounding the cyst in 27.8% of cases, internal septation was observed in 22.2% of cases, and a "cyst within a cyst" appearance, the most common US finding, occurred in 72.2% of cases. Similarly, Lee et al. studied 22 children with abdominal cystic masses and found that the "daughter cyst sign" had a sensitivity of 82% and specificity of 100% for diagnosing follicular cysts (16), highlighting US's capability to readily differentiate this cyst type from others, particularly perinatal torsioned ovarian cysts, by their multilayered walls.
Besides ovarian cysts, the gastrointestinal tract is another significant source of abdominal cysts, which may sometimes be associated with bowel obstruction. Tiwari et al.'s 2017 study evaluated 14 children with gastrointestinal cysts, identifying seven mesenteric cysts, four duplication cysts, and three omental cysts. In 11 patients, the radiologic diagnosis aligned with the final diagnosis, whereas in three patients, only acute intestinal obstruction was initially diagnosed before surgery; all three were later found to have mesenteric cysts (17). Echoing these findings, our study indicated a sensitivity range of 66.6 - 100% and specificity of 94 - 98% for US in diagnosing various gastrointestinal cysts, including duplication cysts, mesenteric cysts, and pancreatic pseudocysts.
In our study, intestinal duplication cysts were identified as the most common diagnosis following ovarian cystic masses, found in 19 patients. Of these, 17 were accurately diagnosed through initial US, yielding a sensitivity of 89.5% and a specificity of 94.1%. We noted that 89% of these cysts presented with a multilayered appearance, and 50% contained debris, aligning with the characteristic ultrasonographic features of duplication cysts. Similarly, Sharma et al. analyzed six cases of enteric duplication cysts, five of which were correctly identified through a combination of US and CT scans before surgery (18). However, the diagnostic accuracy of US alone for identifying intestinal duplication cysts has not yet been established, suggesting the need for further research.
Other cysts arising from the gastrointestinal tract, such as pancreatic pseudocysts and mesenteric cysts, were encountered less frequently than intestinal duplication cysts. Compared to intestinal duplication cysts, these lesions tended to occur in older pediatric patients. Pancreatic pseudocysts in definitive cases had debris in 82% of instances, whereas less than half of the mesenteric cysts (44%) contained debris; moreover, neither type of lesion exhibited multilayered walls. This observation is consistent with the typical characterization of pancreatic pseudocysts as having well-defined fibrous walls and containing necrotic debris during early stages (19, 20).
Regarding the diagnosis of pancreatic pseudocysts, earlier studies indicated that US's sensitivity ranges from 70 - 90% for these lesions, which is lower than CT's sensitivity of 90 - 100% (21). Conversely, our results showed that US was highly sensitive (100%) in differentiating pancreatic pseudocysts from other cystic abdominal lesions. It is important to note, however, that our study focused exclusively on pediatric patients, for whom we could utilize high-frequency probes for retroperitoneal assessments. These probes might not be as effective in adult populations, and cystic lesions could be obscured by intestinal gas in some cases, potentially diminishing the overall sensitivity of US for pancreatic pseudocysts. Additionally, employing graded compression US examinations, sedating children when necessary, and ensuring visualization of the entire pancreas helped to minimize missed cases in our study. Despite these considerations, US proved to be a valuable tool in distinguishing pancreatic pseudocysts from other abdominal cysts in children.
Mesenteric cysts are uncommon cystic lesions encompassing a broad range of differential diagnoses. Etiologically, they can be classified into mesothelial (peritoneal simple mesothelial cyst), lymphatic (simple lymphatic cyst and lymphangioma), enteric, etc. Typically, they manifest with symptoms such as abdominal pain, vomiting, and occasionally intestinal volvulus. On US, they appear as cystic masses characterized by thin walls and internal septations (22). In our study, 78% of mesenteric cysts exhibited internal septations, and 44% contained debris. Among the potential diagnoses, US demonstrated the lowest sensitivity for mesenteric cysts, distinguishing these cysts with a sensitivity of 66.6% and a specificity of 98.1%. In contrast, Parkash et al., who examined 17 children with mesenteric cysts using US, were unable to conclusively diagnose any cases, with definitive diagnoses being made through histopathology post-surgery (23). This underscores the operator-dependent nature of US and its variable sensitivity for specific diagnoses. We also identified a case of cystic mesothelioma, which presented as a multilobular cyst with internal debris and septation adjacent to a solid mass (the ovary). US is the preferred initial evaluation tool for cystic mesotheliomas, typically presenting as multiloculated cysts with anechoic, hemorrhagic, or gelatinous content; often, these are described as having a "spider-in-web" appearance on US due to the presence of an ovary encircled by cystic mesothelioma of the peritoneum (24).
Beyond these common lesions, we occasionally encountered lesions for which the diagnostic value of US could not be assessed. These included sacrococcygeal teratoma cysts, sclerosing encapsulating peritonitis (SEP), urachal cysts, Meckel’s diverticulum, and cystic mesothelioma. Abdominopelvic cystic teratomas in all six cases showed both solid and cystic components, with internal septation, and 66.7% also presented with calcification. These tumors display a diverse range of appearances and may contain fat and calcification; while they can be both cystic and solid, only a small percentage are entirely cystic (19). Teratomas can occur in various locations, including entirely intra-abdominal ones, necessitating differentiation from GI tract-associated cysts or ovarian cysts. A previous study reported a 79.3% positive predictive value of US for teratomas, while recommending further magnetic resonance imaging to assess fetal complications. Our findings regarding these types of cysts remain inconclusive due to their relatively low occurrence in our study group; this is consistent with their incidence of 1 in 35,000 live births (25).
In our study, we encountered three cases of SEP, typically presented as cystic lesions with internal septations, and one patient (33.3%) exhibited a surrounding fibrous membrane. The literature commonly describes SEP with ultrasonographic features such as dilated bowels encased by fibrous membranes, abdominal fluid, and a thickened peritoneal layer (24, 26). Furthermore, two patients presented with urachal cysts, which appeared on US as midline cysts with internal septation and debris.
Additionally, we identified a patient with Meckel’s diverticulum, which presented as a cystic lesion with a multilayered and calcified wall resembling a duplication cyst—an atypical presentation for this condition. US is reported to be highly sensitive (93.6%) and specific (98.1%) in diagnosing Meckel’s diverticulum, attributed to its distinctive appearance. The ultrasonographic findings for Meckel’s diverticulum typically include a single blind-ended thick-walled (> 3 mm) structure connected to the normal intestine at the other end (6).
Contrary to some previous studies, US in our study could provide a conclusive diagnosis for 80.8% of intra-abdominal cysts before surgical intervention. This may be attributed to technical advancements in US technology, including the use of high-resolution multifrequency probes tailored for children, combined with the enhanced experience and knowledge of radiologists, which have improved the sensitivity and specificity of this technique. It is noteworthy that other imaging modalities, such as CT scans, may not reveal the precise details of cysts due to inherent limitations in spatial resolution. Given its high specificity, US can be relied upon to differentiate lesions from one another, potentially reducing the necessity for further imaging studies. Moreover, the radiation-free nature of US aligns well with the "as low as reasonably practicable" (ALARP) principle in radiology safety, emphasizing its suitability, especially in pediatric imaging.
In this study, the limited sample size and inconsistent findings regarding the diagnostic value of US (US) compared to similar studies constitute significant limitations. Although the study included patients under 19 years old, it was conducted in tertiary pediatric hospitals, and older children, especially females with pelvic cysts, may initially consult other outpatient clinics.
Another challenge was the limited access to MRI in our pediatric hospitals. Future studies with a multi-centered design, larger sample sizes, and correlational analyses are necessary.
5.1. Conclusions
Cystic abdominal masses not originating from the liver, kidney, or spleen represent a broad spectrum of pathologic differential diagnoses with varied clinical presentations in children. Preoperative diagnosis has become increasingly common, with US serving as the preferred initial diagnostic tool for evaluating these lesions. In our study, US findings were consistent with the final diagnosis or included in the differential diagnosis in 80.8% of cases, underscoring its reliability for assessing cystic masses in pediatric patients. The study's outcomes suggest that focusing on specific US features can lead to comprehensive and conclusive imaging diagnoses preoperatively, reducing the need for additional diagnostic methods in many cases of cystic abdominal lesions.