1. Background
The incidence of type 1 diabetes in children worldwide has been increasing in recent years (1). Although there is limited data on the incidence of type 1 diabetes mellitus in Iran, studies have reported an increasing trend in different regions of the country (2). This finding highlights the importance of managing and providing care for these children to prevent both macrovascular and microvascular complications. Good control of diabetes reduces the risk of vascular complications (1, 3, 4). Chronic hyperglycemia in diabetic patients is closely associated with long-term damage to various organs, including the eyes, kidneys, nerves, heart, and blood vessels (5). Diabetic nephropathy (DN) is a microvascular complication that occurs in 20-40% of patients (6) and can lead to end-stage renal disease (7, 8).
The initial stage of DN involves the presence of abnormal levels of albumin in the urine, known as microalbuminuria, which typically ranges from 30-299 mg per day (9). This condition arises due to abnormally high permeability to albumin in the kidney’s glomeruli (10). Microalbuminuria is used to diagnose nephropathy and occurs when albumin levels within the range of 30 to 300 mg are found in a 24-hour urine collection (10).
Previous studies have established a positive connection between microalbuminuria and vascular complications in diabetic patients (11-13). Different studies have explored the impact of various risk factors, such as glycemic control, overweight, and duration of diabetes, on the occurrence of microalbuminuria, yielding diverse results (14, 15). Consequently, further investigations are required to elucidate these risk factors. The early identification and treatment of these risk factors can help prevent DN and other micro and macrovascular complications.
2. Objectives
The aim of this study was to examine microalbuminuria in children with type 1 diabetes and assess its relationship with body mass index (BMI), hemoglobin A1c (HbA1c) levels, and the age of diabetes onset. Type 1 diabetes mellitus (T1D).
3. Methods
This cross-sectional study was conducted on children with type 1 diabetes over a 1-year period within February 2021 to March 2022 in Tehran, Iran. Participants were selected using convenience sampling. Children and adolescents with type 1 diabetes (DM1) who were referred to the Pediatric Endocrinology Clinic of Imam Hossein and Mofid hospitals were included in this study. The diagnosis of DM1 was made based on the criteria of the American Diabetes Association (ADA).
According to Jim Frost (16), the smaller sample size was determined based on the number of observations needed to achieve a statistical power of 0.80, which falls between 100 and 120 observations. Based on the inclusion criteria, a total of 120 patients with DM1, aged between 5 and 20 years, were included in the study. This study received approval from the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.MSP.REC.1399.720).
The inclusion criteria consisted of individuals aged 5 to 20 years, with type 1 diabetes for a minimum of 3 years, and receiving treatment with subcutaneous insulin (neutral protamine Hagedorn [NPH] and regular or recombinant insulin). The exclusion criteria included low-density lipoprotein (LDL) and/or triglyceride levels > 97th percentile for age and gender, urinary system disorders, smoking, use of angiotensin-converting enzyme inhibitor (ACE) inhibitor drugs, history of macroalbuminuria, blood pressure > 90th percentile for age and gender, known kidney scarring, and a lack of consent to participate in the study.
After obtaining consent from the patients or their parents, the patients were enrolled in the study. To record patient information, a checklist was designed, and the data regarding age, gender, age of onset of diabetes mellitus, and disease duration were collected. Additionally, measurements of weight, height, and blood pressure were taken using standard instruments in kilograms (kg), meters (m), and millimeters of mercury (mmHg), respectively. Weight was measured using a Seca scale (Germany), height was measured with a wall-mounted meter, and blood pressure was recorded with a suitable Riester sphygmomanometer (Germany) after the patient had rested for 5 minutes.
Body mass index was calculated using the following formula:
(weight) kg / (height) m2
Hemoglobin A1c and random urine tests for microalbumin and creatinine were requested for the patients simultaneously and then analyzed in the reference laboratory, with the results recorded in the form. Blood samples were collected from all subjects between 8 a.m. and 9 a.m. in the reference laboratory. Hemoglobin A1c levels were measured using the high-performance liquid chromatography (HPLC) method with Labnovation LD-600 equipment (China). Urine albumin was assessed using the immunoturbidimetry method with Prestige 24i equipment (Japan). Mid-morning urine samples were used to determine the urine albumin levels, and urine creatinine was measured enzymatically in the reference laboratory. Microalbuminuria was defined as a urine albumin-to-creatinine ratio within the range of 30 - 299 milligrams per gram of creatinine, confirmed in two tests within 6 months. Levels exceeding this range were classified as macroalbuminuria (17, 18). Cases that could potentially yield false positive results were excluded from the study. These cases included individuals with urinary tract infections, high fever due to infection, strenuous exercise before the test, the use of certain drugs, such as aspirin, corticosteroids, or certain antibiotics, such as amoxicillin, menstruation, or vaginal discharge (19).
3.1. Statistical Analysis
The collected data were entered into SPSS software (version 19) for analysis. Descriptive statistics, such as mean, standard deviation, and median, were used to describe the data. Initially, the data were assessed for normality, and the Johnson transformation was applied to normalize the data distribution.
The Johnson transformation formula is as follows:
- 0.941703 + 0.648995 * ASINH ([X - 4.08963] / 2.4715)
Data analysis was carried out by stepwise regression analysis, in which the relationship between albumin secretion values with HbA1c level, age at the onset of the disease, and BMI was investigated. A p-value of less than 0.05 was considered a significant level.
4. Results
A total of 120 cases, aged between 5 and 20 years with a mean age of 13 years, were included in the study. Out of these 120 cases, 61 (51%) and 59 (49%) patients were female and male, respectively. The average duration of their disease was 5.5 years, ranging from 3 to 15 years. Regarding their BMI status, 34 (28.3%), 70 (58.3%), 13 (10.8%), and 3 (2.5%) patients were classified as underweight (BMI less than the 5th percentile for age and gender), normal (BMI ranging from the 5th percentile to less than the 85th percentile), overweight (BMI ranging from the 85th to less than the 95th percentile for age and gender), and obese (BMI above the 95th percentile for age and gender), respectively (20).
The urinary albumin excretion levels were distributed in 101 (84.1%), 18 (15%), and 1 (0.8%) patients with levels below 30, 30-299 mg, and over 300 mg, respectively. Based on their HbA1c levels, 11 (9.1%), 41 (34.1%), and 68 (56.6%) patients exhibited good control (HbA1c less than 7%), unfavorable control (HbA1c between 7% and 9%), and poor control of diabetes (HbA1c above 9%), respectively. A significant relationship was observed between microalbuminuria and HbA1c levels (P = 0.002).
The results presented in Tables 1 and 2 show the relationship between demographic, BMI, and laboratory data with microalbuminuria through univariate and multivariate regression analyses, respectively.
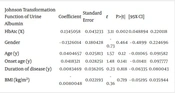
| Johnson Transformation Function of Urine Albumin | Coefficient | Standard Error | t | P>|t| | [95% CI] | |
|---|---|---|---|---|---|---|
| HbA1c (%) | 0.1345058 | 0.043233 | 3.11 | 0.002 | 0.048894 | 0.220118 |
| Gender | - 0.1326014 | 0.180428 | - 0.73 | 0.464 | - 0.4899 | 0.224696 |
| Age (y) | 0.0404657 | 0.025813 | 1.57 | 0.12 | - 0.01065 | 0.091582 |
| Onset age (y) | 0.0418321 | 0.028251 | 1.48 | 0.141 | - 0.01411 | 0.097777 |
| Duration of disease (y) | 0.0083469 | 0.036205 | 0.23 | 0.818 | - 0.06335 | 0.080043 |
| BMI (kg/m2) | - 0.0080048 | 0.022193 | - 0.36 | 0.719 | - 0.05195 | 0.035944 |
Abbreviations: HbA1c, hemoglobin A1c; BMI, body mass index.
| Johnson Transformation Function of Urine Albumin | Coefficient | Standard Error | t | P>|t| | [95% CI] | |
|---|---|---|---|---|---|---|
| Gender | - 0.00292 | 0.174341 | - 0.02 | 0.987 | - 0.34832 | 0.342476 |
| Onset age (y) | 0.091692 | 0.036921 | 2.48 | 0.014 | 0.018544 | 0.16484 |
| Duration of disease (y) | 0.024811 | 0.036934 | 0.67 | 0.503 | - 0.04836 | 0.097983 |
| BMI (kg/m2) | - 0.05404 | 0.027776 | - 1.95 | 0.054 | - 0.10907 | 0.000992 |
| HbA1c (%) | 0.134086 | 0.043464 | 3.08 | 0.003 | 0.047975 | 0.220196 |
Abbreviations: HbA1c, hemoglobin A1c; BMI, body mass index.
In the multivariate model, a significant relationship was observed between the age of onset (P = 0.014), BMI (P = 0.054), and HbA1c (P = 0.003) with microalbuminuria.
5. Discussion
In this descriptive cross-sectional study, 120 children and adolescents with type 1 diabetes, aged between 5 and 20 years, were evaluated, with 51% of them being female. Among all patients, 2.5%, 10.8%, and 58.3% were classified as obese, overweight, and normal, respectively. Microalbuminuria was observed in 15% of the patients, and macroalbuminuria was detected in only 1 patient (0.8%). A significant relationship was observed between the age of diabetes onset, BMI, and HbA1c levels with microalbuminuria.
In line with the present study, a similar prevalence of microalbuminuria (15%) was reported in a study conducted on diabetic patients in Kuwait (21). Favel et al.’s study showed that 11% of children with type 1 diabetes had abnormal urine albumin-to-creatinine ratios (> 2.5 mg/mmol) (22). The aforementioned results indicate a higher prevalence than reports from some populations, such as 3.3% and 5% in the USA and Germany, respectively (23, 24). However, the prevalence was comparable to that in some other countries, such as 13% of West Australian children with T1DM and 13.4% of Indian children (25, 26). In explaining these differences, in addition to genetic variations, factors related to the study population and accompanying risk factors should be considered. For instance, in the present study, more than 56% of patients had poor glycemic control (HbA1c > 9%).
Diabetic nephropathy encompasses different stages, including glomerular hyperfiltration with normal albuminuria, early nephropathy with microalbuminuria, overt nephropathy or macroalbuminuria, and, ultimately, end-stage renal disease (15, 17). Microalbuminuria assessment is the gold standard method for detecting DN (27). It is suggested that factors such as urinary tract infections, acute illnesses, and exercise can cause nonspecific microalbuminuria (15). In the current study, patients with a history of severe exercise and/or acute illnesses were excluded because these conditions can lead to false positives in the albuminuria test.
In Schultz et al.’s study, they reported that the occurrence of microalbuminuria within the first 5 years of diabetes onset is low; however, it significantly increases with the onset of puberty (28). This increase might be attributed to various factors, including the rise in sex hormones and growth hormones, insulin resistance, and reduced adherence to the treatment plan during puberty (28).
Hemoglobin A1c has been identified as the most critical risk factor for renal involvement in most studies (15, 29). In the present study, consistent with the findings of Huang et al., glycemic control exhibited the highest correlation with DN. Effective blood sugar control reduces the incidence of microvascular complications in patients (29). Frequent blood sugar monitoring, carbohydrate counting education, and adjusting insulin doses based on carbohydrate intake contribute to proper glycemic control. Real-time continuous glucose monitoring technology offers the potential for precise blood sugar regulation without inducing hypoglycemia (14).
Although Huang et al.’s study, in addition to German and Swiss studies, identified male gender as a risk factor for DN (29, 30), a study conducted in the USA reported a higher prevalence of DN in female individuals (23). In the present study, similar to Razavi et al.’s study, no significant gender-based differences in the risk of DN were observed (31). These variations in results might be attributed to genetic or racial differences.
The present study demonstrated that an increasing BMI was associated with a higher risk of microalbuminuria, consistent with the findings of Ahmadi’s, Chaturvedi’s, and Zabeen’s studies (32-34). Ahmadi’s study reported that a BMI > 30 kg/m2 was a risk factor for renal damage (32). Ramaphane et al. discovered significant associations between gender, diabetes duration, HbA1c, and microalbuminuria in diabetic children but no association with BMI and microalbuminuria (15). The link between obesity and microalbuminuria in diabetic patients might be due to the coexistence of insulin resistance (33). Insulin resistance can directly impact endothelial damage and the development of microalbuminuria. This damage might arise from reduced insulin action and increased capillary albumin leakage (33).
In the current study, significant associations were observed between the age of disease onset, HbA1c levels, and BMI with microalbuminuria; nevertheless, no association was observed between gender and disease duration with albuminuria. Longitudinal studies are probably more effective in investigating the relationship between disease duration and the prevalence of microalbuminuria.
It should be noted that the present study lacked information regarding the participants’ puberty status and stage, and it was not possible to follow up with diabetic patients who developed microalbuminuria.
5.1. Conclusions
Based on the results of this study, significant relationships were observed between microalbuminuria, an indicator of microvascular complications, and both HbA1c levels and BMI. The current study also indicated that an early onset of diabetes was a risk factor for microalbuminuria in diabetic children. It is suggested to consider early screening for diabetic patients with these risk factors in the prevention of microvascular complications.