1. Background
In 2017, the World Health Organization (WHO) requested that different countries implement strategic plans and necessary measures to prevent and control hearing loss and decrease the burden of disease. According to the WHO, 466 million people around the world suffer from hearing loss, of whom 34 million are children (1, 2). In 2014, Iran's Non-Communicable Diseases Management Office compiled the first national guidelines for hearing impairment screening in infants and children and established the National Hearing Health Committee of the Ministry of Health and Medical Education in cooperation with the State Welfare Organization of Iran. Considering the necessity of updating the mentioned guidelines and also in order to integrate Ear and Hearing Healthcare (EHC) into the public health network, these guidelines were reviewed by experts of the Non-Communicable Diseases Management Office with the cooperation of the National Committee on EHC, Primary Health Association (PHC), and other organizations such as the Iranian societies of Otolaryngology, Head and Neck Surgery, Audiology, Pediatrics, and General Practitioners. In this way, a national program for hearing screening care of infants and children was finally implemented by all medical universities in the country in the framework of the primary health care system.
Otoacoustic emissions (OAE) and automated auditory brainstem response (AABR) represent two practical and non-invasive methods grounded in electrophysiological concepts, serving as primary screening tools in healthcare centers across Iran (3). OAE involves tracking sounds emitted by the cochlea when it is stimulated acoustically. The transient evoked otoacoustic emission (TEOAE) test, an automated procedure, is utilized to assess hearing capabilities. It marks the initial phase of auditory screening for all newborns, irrespective of the presence or absence of risk factors for hearing loss at birth. Similarly, AABR constitutes the subsequent screening step for evaluating the sensorineural hearing condition of newborns. Conducted in an entirely automated fashion, akin to OAE, this test applies to click stimuli at an intensity range of 35 to 40 dB. It examines the auditory nerve (also known as the eighth cranial nerve) and the higher segments of the brainstem, determining the existence or non-existence of the fifth wave in the brainstem's auditory potentials in reaction to specific click stimuli thresholds. The outcomes of this screening are automatically produced on a device, indicating either "Refer" or "Pass" as the result (4-7).
Iran's newborn hearing screening program is carried out in two settings: Within hospitals and/or at tertiary health centers. The national newborn hearing screening protocol is outlined as follows:
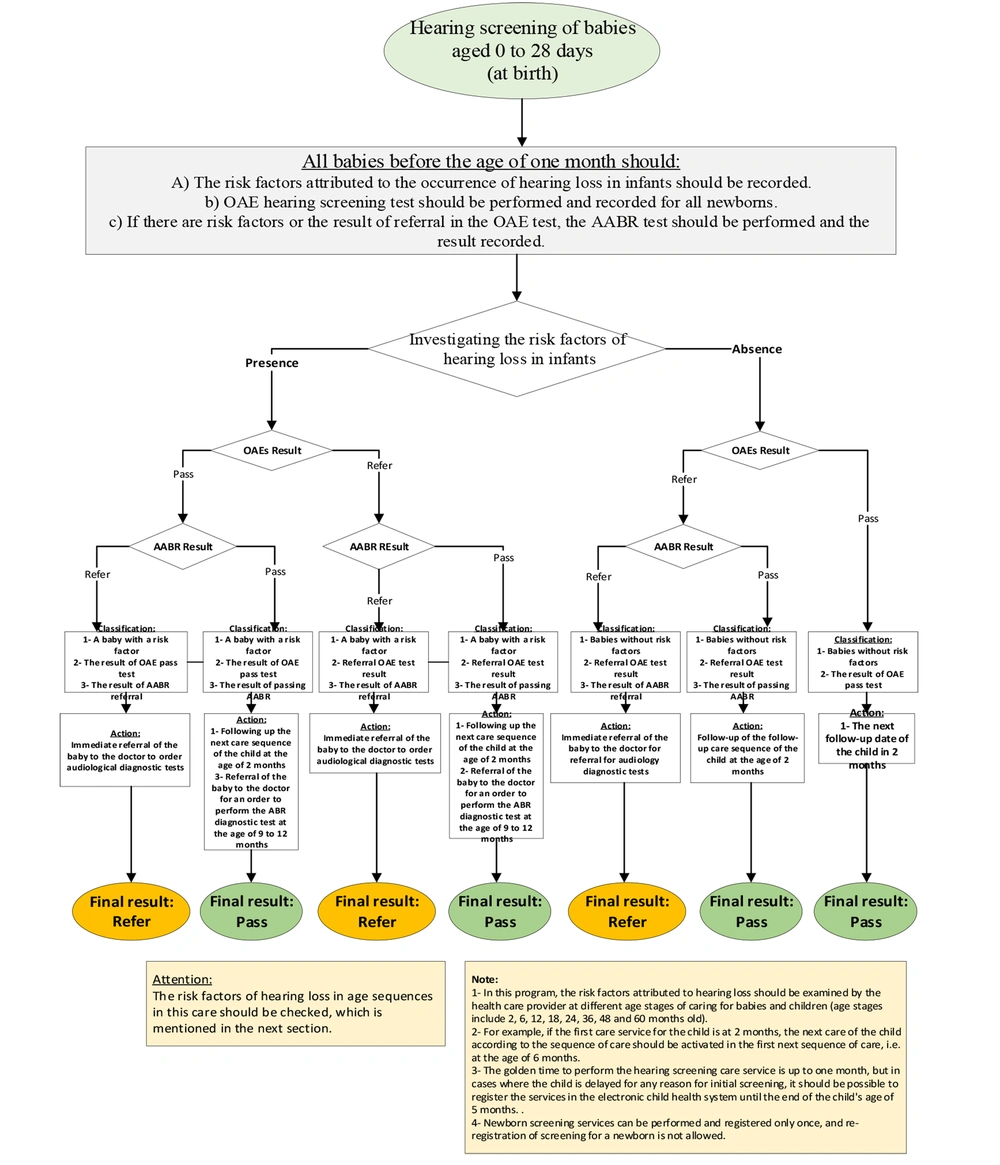
1- Initially, the OAE test is administered to newborns aged 0 - 28 days. If the test yields a "referral" outcome for one or both ears or if at least one risk factor for hearing issues is identified, the AABR test is conducted either in the same session or as promptly as possible.
2- Infants who receive a referral result, either unilateral or bilateral, from the second screening test (i.e., AABR) must be directed to the closest audiology clinic in their living area to undergo a sequence of confirmatory audiological diagnostic evaluations.
3- The outcomes of the audiological diagnostic and screening referral tests should be documented within four weeks following the second screening (i.e. before the infant reaches three months of age). Confirmation of unilateral or bilateral hearing loss should be made as soon as feasible, within a week. Subsequently, the infant should be referred to an otolaryngologist or an audiologist for the necessary treatment/rehabilitation interventions.
The cost-effectiveness and efficiency of hearing screening programs are critical determinants of their success. However, the cost-effectiveness of these programs has been insufficiently explored to date (8, 9), particularly in less-developed and developing countries. Moreover, due to the variety of protocols employed in different nations, the Incremental Cost Effectiveness Ratio (ICER) values reported in these studies exhibit broad variation. For instance, Uus et al. analyzed the cost-effectiveness of universal screening (based on OAE) versus targeted screening (based on AABR) within the framework of newborn hearing screening (NHS) programs. In Iran, OAE is predominantly utilized for hearing screening as part of national health initiatives (10). A study in Australia found that universal NHS costs an average of 22,000 Australian dollars (A$) per child, yielding an additional 0.45 Quality Adjusted Life Years (QALYs) per diagnosed child. Conversely, targeted screening over up to five years presented an incremental cost-effectiveness ratio of A$48,000 per QALY (9).
2. Objectives
Given the widespread prevalence of hearing impairments, the constrained resources at both national and international levels for providing high-quality services to newborns with hearing disorders, and the potential consequences of such impairments, we aimed to conduct a cost-effectiveness analysis (CEA) on the prevailing screening methods (AABR and OAE, as central elements of the NHS program) in Iran, using data from electronic health systems at the primary level of the referral chain within the Ear and EHC Management National Program, under the NCD Office, MOHME, I.R. Iran.
3. Methods
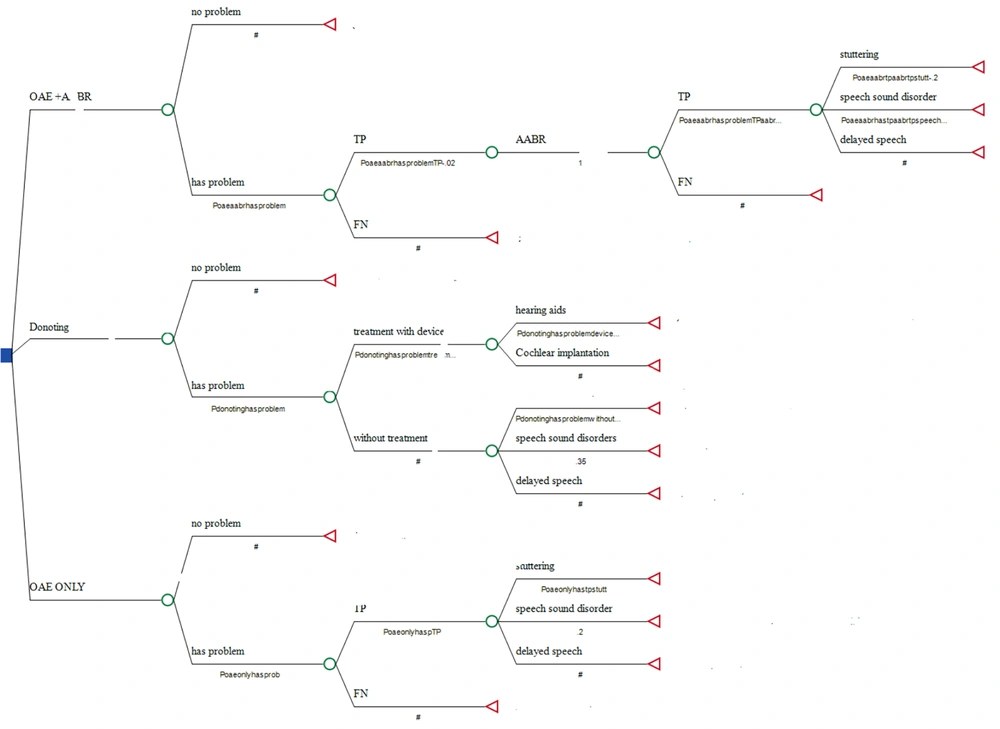
The deployment of a two-step hearing screening protocol is designed to diminish the rate of false positives and enhance the effectiveness of the country's newborn hearing screening program. We assessed the cost-effectiveness of this two-tier national NHS program in Iran, as illustrated in Figure 1. Figure 1 delineates three strategies within the ambit of Iran's NHS program. The primary strategy involves the use of both OAE and AABR tests. In this context, an infant is either diagnosed with a hearing condition or not. Should there be no issue, the process concludes; however, if an issue is identified, two outcomes are possible: A true positive or a false negative, determined by the test's specificity and sensitivity. In the event of a true positive, the procedure advances to the AABR test. Alternate pathways within this model are depicted in Figures 1 and 2.
In the second strategy, TEOAE is utilized to initially screen all newborns, with those failing or passing marginally being referred to selected hospitals for a reevaluation using OAE within two weeks after the initial assessment. Newborns unable to pass this second evaluation are then referred to another specialized center for an AABR test, the results of which are used to establish a final diagnosis. While healthy newborns are recommended to undergo a repeat evaluation within six months, those diagnosed with hearing impairments are directed to auditory training and rehabilitation centers. For the cost-effectiveness analysis of these hearing loss screening strategies, we employed TreeAge (version 2022) software.
The newborn hearing screening program is implemented at the primary level of health services within hospitals offering maternity services to pregnant women, as well as in selected comprehensive health centers. We gathered data on 1,006,293 newborns (from a total of 1,106,072 live births) through the electronic health system, representing the primary level of the referral system of the Ear and EHC Management National Program, under the NCD Office, MOHME, I.R. Iran. This data was documented on specific forms devised by the EHC Management National Program, NCD Office, encompassing demographic details (such as gender, age, risk factors, etc.), initial screening and confirmatory OAE results, and outcomes (namely, referrals for outpatient rescreening). Evaluators filled out these data forms. In Iran, information on newborns' hearing screenings, including potential risk factors and the results of the OAE-AABR tests reported by audiologists, is recorded by trained health workers in the electronic health systems at the primary level of the referral system.
3.1. Cost Estimation
Resource utilization data were collected retrospectively from the Ministry of Health (MoH) and centers conducting AABR screenings for hearing loss. Costs were calculated from a governmental perspective. In Iran, the State Welfare Organization (SWO) subsidizes certain expenses, such as the acquisition of screening equipment, while families are responsible for covering governmental fees for screening services. Additionally, private sector entities are mandated to provide newborn hearing screening services at rates established by the MoH. For the purpose of assessing the program's cost-effectiveness, the expenses borne by families for screening tests were considered the actual costs.
The primary components of these costs included (a) fixed costs, such as expenses for screening equipment (TEOAE and ABR sets), and (b) variable costs, like the charges for screening tests (OAE), excluding indirect expenses, such as transportation and overhead. Estimates included patient user fees and data from databases of other contributors like the Welfare Organization and hospitals. Actual expenditures incurred by various organizations were compiled. Direct payments to screeners or staff were not applicable, as salaries and expenses for maintenance and consumables are included in the OAE and AABR service charges. Hence, these costs were not separately accounted for. Additionally, no transportation costs were considered during initial hospital screenings, with patient payments solely for ABR rescreening. Consequently, these and long-term costs were excluded from the cost calculation. All expenses, calculated in Iranian Rial (IRR), were viewed from the health system's perspective and converted to US dollars based on the exchange rate from the International Monetary Fund (IMF). The treatment for speech disorders was anticipated to need between 21 and 24 sessions, each lasting between 18.5 and 26.5 hours. Some of these costs are detailed in Table 1.
| Parameters | AABR | OAE |
|---|---|---|
| Device purchase | 4503 - 6163 | 2768 - 3574 |
| Annual device repair and maintenance | 135 - 185 | 89 - 115 |
| Monthly salaries of human resources | 368 - 450 | 368 - 450 |
Abbreviations: AABR, automated auditory brainstem response; OAE, otoacoustic emissions.
3.2. Outcomes
In this study, Quality of Life (QoL) and QALYs were used as outcomes. QALYs, which are time-based measures, and health-related QoL scores quantify the quality of an individual's life on a scale from 0 to 1.00, where 0 represents death, and 1.00 signifies optimal health. A QoL utility score is transformed into a QALY by multiplying the utility value by the number of years lived with that level of health quality. The methodological validity of employing QoL utility as a denominator in cost-effectiveness analysis relies on four premises:
• Society shares a unified perception of what constitutes perfect health.
• It is possible to quantify less-than-perfect health on a linear utility scale in comparison to death and perfect health.
• Health conditions of lesser quality are as quantifiable as those of higher quality.
• Societal value is equally attributed to positive and negative changes in health status that have identical magnitudes on the utility-scale.
The outcomes were established based on an exhaustive study titled "Measuring quality of life in children with speech and language difficulties: A systematic review." Additional parameters were determined by the authors, as shown in Table 2.
| Parameters | AABR | OAE |
|---|---|---|
| Sensitivity | 0.93 | 0.77 |
| Specificity | 0.97 | 0.93 |
| Prevalence of hearing loss | 3 per 1000 live births | |
| Annual birth rates | One million newborns ± 11% | |
| Average costs per newborn (without a share of insurance) | 20.17 | 55.83 |
Abbreviations: AABR, automated auditory brainstem response; OAE, otoacoustic emissions.
3.3. Sensitivity Analysis
The robustness of the cost-effectiveness modeling results was evaluated through one-way sensitivity analysis. Given the modeling assumptions or requirements of the program, certain parameters may vary in both outcomes and costs. A variation of ±20% was applied to the target variables and the estimated costs per identification. In this study, Monte Carlo simulation was employed for sensitivity analysis, necessitating the use of parameter distributions (Table 3).
| Parameters | Distribution | Properties of Distribution |
|---|---|---|
| Probabilities: The probabilities of hearing loss in newborns and the probability of not being treated and developing related diseases such as stuttering, speech sound disorder, and delayed speech | Beta | Bounded between 0 and 1. Alpha and Beta values were calculated as follows: Alpha = mean2 × [(1-mean)/SE2] - mean; Beta = Alpha×[(1-mean)/mean] |
| Costs: Hearing aids and physician appointments, hearing aids, physician appointments, appropriate medical treatments, auditory rehabilitation, speech disorders, and delayed speech. | Gamma | Bounded at 0, positively skewed. Derived from mean and its standard error, calculated as follows: Alpha = (mean/SE)2; Beta = SE2/mean; Lambda = mean/SE2 |
| Utility and QALY: Utility and disability weight of hearing disability, speech disorder and delayed speech in children. | Gamma | Bounded between 0 and 1 and based on Miro fit software and calculation of observations |
4. Results
In 2022, Iran witnessed the birth of 1,106,072 babies, and hearing screenings, as part of the NHS program, were conducted for 1,006,293 of them, yielding a coverage rate of 90.97%. The findings revealed that 3,359 newborns experienced hearing issues, translating to a hearing loss prevalence of 3 per 1,000 live births (Table 4).
| Total Births | Hearing Screening Coverage | Case Detected | Female Newborn Detected | Male Newborns Detected |
|---|---|---|---|---|
| 1106072 | 1006293 (90) | 3359 (3per1000 newborn) | 1652 (49) | 1707 (51) |
a Values are presented as No. (%).
The results showed that the OAE plus AABR strategy had the highest cost-effectiveness, recommending this approach as the dominant strategy, followed by OAE alone. As it can be observed, Willingness to Pay (WTP) is three times the GDP per capita.
Table 5 shows incremental cost-effectiveness, where cost changes are defined as changes in effectiveness (QALY). According to this table, the amount of ICER was equal to $ 3297.2 US PPP, which is much less than three times the GDP per capita ($38100) and even the raw GDP, verifying the cost-effectiveness of AABR plus OAE. Finally, the dominant strategy was found to be OAE plus AABR.
| Strategies | Cost Convert to PPP ($) | Cost Iranian Currency (Rial) | Effectiveness (QALY) | ACER (PPP) US $ | ICER (PPP) US $ |
|---|---|---|---|---|---|
| Donating | 4.45 | 133075.31 | 8.6 | 0.51 | - |
| OAE + AABR | 4126 | 122560000 | 9.81 | 420.5 | 3297.2 |
| OAE only | 2548 | 75700000 | 8.75 | 291.2 | 16957 |
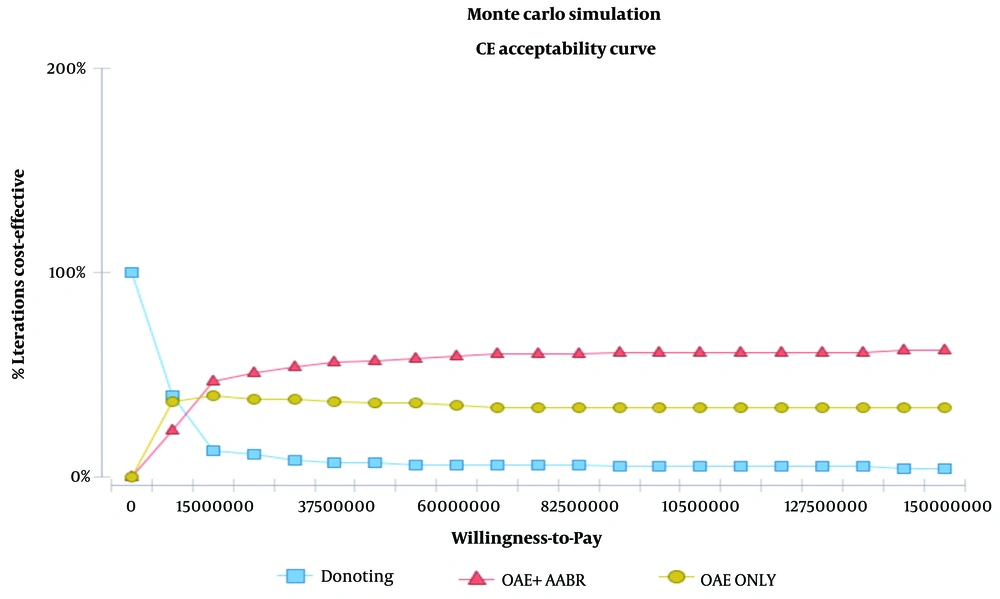
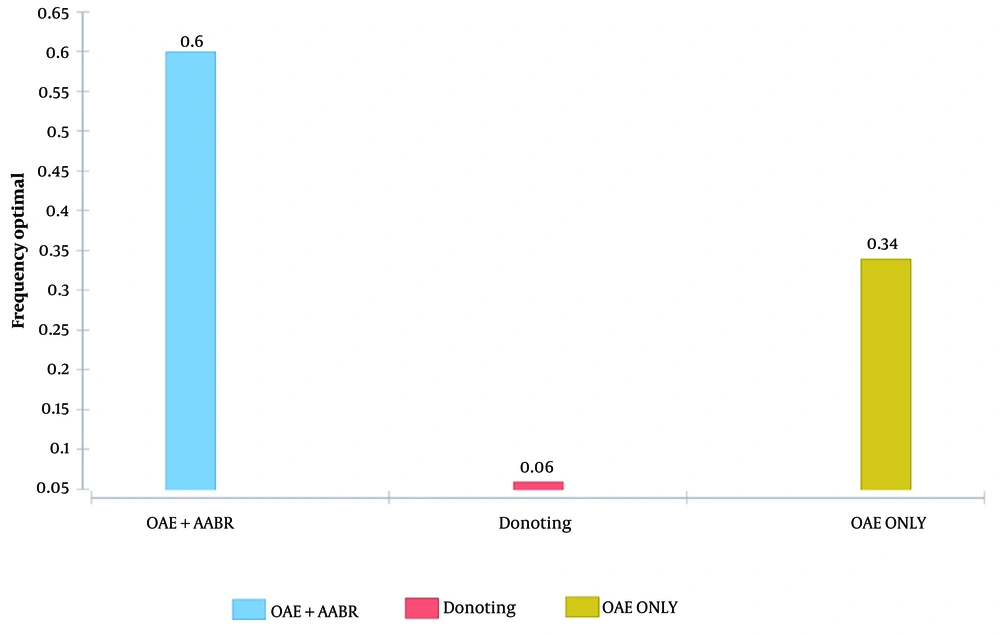
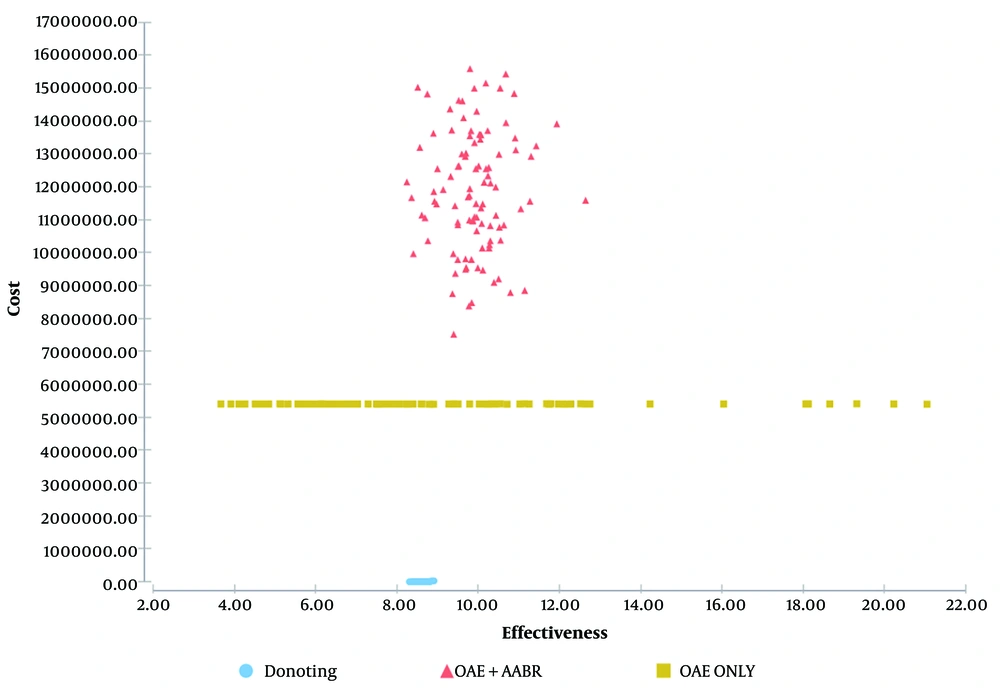
Figure 3 illustrates the probability that one intervention is more cost-effective than the other for a given WTP (Willingness-To-Pay), assuming that OAE + AABR is superior to OAE alone and to no intervention. If decision-makers were willing to pay a maximum of 150,000,000 Iranian Rials or $5049.77 US PPP per effectiveness gained, no intervention would show greater cost-effectiveness (100%) under this assumption. If the WTP was at least 150,000,000 Iranian Rials or $5049.77 US PPP per effectiveness gained, OAE + AABR was more cost-effective with a probability of 60%. Furthermore, Figure 3 demonstrates that as the WTP increases, the OAE + AABR strategy becomes more cost-effective. According to Figure 2, the OAE + AABR strategy was cost-effective, with a probability of 60%. After 1000 iterations, the second most cost-effective strategy was found to be OAE alone. Additionally, the cost-effectiveness scatter plot obtained by the Monte Carlo simulation model (Figure 4) showed that costs would be lower when OAE was used as the only intervention.
4.1. Sensitivity Analysis
The two-way sensitivity analysis demonstrated that the magnitude of each risk factor followed a descending order, resembling a tornado-shaped funnel. These tools, recognized as suitable project management tools, provide valuable data for decision-making and risk evaluation at various stages of a project. The sensitivity analysis confirmed the effectiveness of OAE + AABR and indicated that the costs of OAE had the most significant influence on its cost-effectiveness results. However, altering these parameters did not affect the outcomes (see Figure 5). In the sensitivity analysis, uncertainty intervals were examined based on the willingness-to-pay threshold, revealing that the ICER value remained below this threshold even after adjusting the desired parameters within a certain range.
5. Discussion
Hearing loss and hearing impairment are growing problems in developing countries, including Iran. The prevalence of congenital hearing problems in newborns reaches 0.5 - 6 per 1000 live births, highlighting the significant role of screening programs in early detection (11). Nowadays, limitations in funding health agencies by governments have hindered preventive health screening programs, especially concerning newborn hearing loss screening (12). Studies investigating hearing problems in infants are limited in Iran, with most available studies conducted on small sample sizes, limiting the generalizability of their results. In this study, we aimed to investigate the cost-effectiveness of the NHS program in Iran by analyzing data provided by the Ministry of Health of Iran. In 2022, 1,106,072 babies were born in Iran, and a hearing test was performed for 1,006,293 of them, showing that more than 90% of the babies were covered by this program. Among these, 3359 infants were diagnosed with hearing problems, indicating a prevalence of 3 per 1000 births.
The scrutiny and comparison of various interventional plans in terms of their health outcomes and costs can be effectively performed through cost-effectiveness analysis, comparing them regarding associated costs for achieving every unit of a health outcome, like a QALY or detecting a case with hearing problems. In this study, QALY was considered as the outcome measure. According to this study, the amount of ICER was equal to $3297.2 US PPP for the AABR + OAE strategy and $16,957 US PPP for OAE alone, showing that the AABR + OAE strategy had an ICER less than three times the GDP per capita ($38,100), indicating that AABR plus OAE was more cost-effective than OAE and should be regarded as the dominant strategy to screen newborns for hearing impairment in Iran.
We found a few studies on the cost-effectiveness analysis of hearing loss screening strategies. In a study in Australia, Sharma et al. investigated the cost-effectiveness of the universal neonatal hearing screening program and reported that this strategy led to an ICER of $48,000 per QALY. Also, the program was considered to be cost-effective at a WTP threshold of $60,000 (9). In another study in Shiraz, Iran, Faramarzi et al. evaluated if screening primary school children for hearing impairment was cost-effective, reporting an ICER of $2.37 PPP for each prevented DALY (13). Yong et al. also reviewed the studies analyzing the cost-effectiveness of hearing loss screening programs in school children and reported that out of five studies, four considered this screening strategy to be cost-effective based on ICERs ranging from $1079 to $4304 (14). In a report from China, the cost-effectiveness of the NHS was assessed in eight provinces, showing that either universal or targeted screening strategies were cost-effective based on various parameters obtained, including calculated ICERs (15). According to our results, the dominant cost-effective strategy for hearing impairment screening was OAE plus AABR. Additionally, willingness to pay increased when the OAE + AABR strategy was offered to parents. Our results also demonstrated that the cost-effectiveness of the OAE + AABR strategy obtained a probability of 60%. After 1000 iterations, the second cost-effective strategy was found to be OAE alone. Accordingly, the Monte Carlo simulation model showed that costs would be lower when OAE was used as the only intervention. As observed, WTP was three times the GDP per capita, and based on the given WTP, OAE + AABR was superior to OAE alone. If decision-makers were willing to pay a maximum of 150,000,000 Iranian Rial (or $5049.77 US PPP per effectiveness gained), no intervention would show more cost-effectiveness (100%). However, for a WTP of at least 150,000,000 Iranian Rials (or $5049.77 US PPP per effectiveness gained), OAE + AABR was more cost-effective with a probability of 60%. Therefore, as WTP increases, the OAE+AABR strategy becomes more cost-effective.
Consistent with our observation, Tobe et al., in a study in China, reported that targeted and universal OAE, followed by universal OAE plus AABR, were the most optimal strategies to promote hearing screening programs. It was suggested that the combination of OAE and AABR could save costs by reducing the rate of false positive cases in the NHS program and delivering higher sensitivity and specificity compared to the strategies using either OAE or AABR alone. Therefore, the OAE plus AABR strategy was suggested as the ultimate approach for detecting hearing loss in newborns (16). Additionally, Jafarlou et al. stated that among 11,168 Iranian newborns screened by OAE alone, 3125 cases required a second testing because of unsuccessful results in the first OAE, and this phenomenon imposed additional expenses on families and the health system (17). In another study in Iran by Heidari et al., AABR alone was performed to detect hearing loss in one million Iranian newborns, identifying 4650 affected cases at a total cost of $3,310,700. In comparison, OAE alone detected 3850 cases for a total cost of $3,414,100, indicating that OAE alone had lower sensitivity and higher costs (18). In contrast, Verkleij et al. reported higher sensitivity for AABR alone compared to AABR + OAE for detecting infants with hearing impairment; however, this higher sensitivity came at higher costs, which is consistent with our findings (19). Overall, the recent study, in accordance with our research, indicated that the two-stage screening (i.e., OAE + AABR) was the most cost-effective strategy regarding the willingness-to-pay threshold proposed by the WHO. Finally, our and other studies' findings support the idea that the combination of OAE and AABR can be the most effective approach to identifying newborns with hearing problems.
5.1. Limitations
This study has several limitations. Firstly, the screening of infants for hearing impairment was performed only once during their first 24 hours of life, while confirmation of hearing impairment generally requires sensitivity screening tests on multiple occasions after birth. Furthermore, an exact estimation of the expenses of NHS programs demands calculating both direct and indirect costs; however, only direct costs were considered in this study. Further studies with a more comprehensive approach toward the indirect costs of these screening programs are recommended as well.
5.2. Conclusions
In this study, we evaluated the cost-effectiveness of Iran's NHS program for detecting hearing impairment by analyzing the data of over a million live births in the country. Using a two-step approach, OAE + AABR was found to be the dominant cost-effective strategy to detect newborns with hearing impairment because it provided more sensitivity and specificity. We recommend estimating both direct and indirect costs in future studies to acquire more precise information on the total expenses of NHS programs. Since AABR + OAE was found to be the most cost-effective method for screening Iranian newborns, it is recommended to implement this strategy as the primary step to early identify and prevent the progression of hearing impairment in Iranian neonates. The use of the combined (i.e., AABR + OAE) strategy for screening hearing loss in newborns, as a more cost-effective strategy than OAE, can lead to early diagnosis of hearing impairments in neonates and save the families, the newborns, and health systems the expenses imposed by future therapeutic interventions. Besides, these newborns can benefit from earlier and timely rehabilitation procedures, which can prevent delays in speech development and school enrollment. Our results provide new insights into the effectiveness of hearing impairment screening methods in newborns. Studies are required to be extended in this area to obtain more comprehensive data on this topic and determine the applicability of different hearing loss screening strategies stratified by socioeconomic classes of Iranians.