1. Background
The history of dextrocardia dates back to the 17th century when it was first reported by Fabricius during a necropsy inspection. Since then, many definitions and classifications have been published to improve the understanding of this condition. In 1964, Van Praagh et al. defined dextrocardia as the positioning of the heart predominantly in the right hemithorax on a plain chest X-ray (1). Today, the most accepted definition of dextrocardia is an intrinsic cardiac malposition where the base-apex axis of the heart is directed toward the right side of the body (2). It should be distinguished from dextroposition, in which the heart is located on the right side with or without a normal axis due to extracardiac factors such as pulmonary, pleural, or diaphragmatic abnormalities (3).
Dextrocardia is often associated with several other cardiac abnormalities, necessitating a thorough and segmental examination to investigate the heart more comprehensively (1, 2, 4). Previous studies have focused on the associated cardiac defects in dextrocardia in both the prenatal and postnatal periods (5-9).
Generally, dextrocardia is classified as situs solitus, situs inversus, or situs ambiguous (also called heterotaxia). Although this classification is useful for categorizing dextrocardia, it lacks sufficient focus on the internal structures of the heart. A morphologic classification based on the cardiac chambers and their interrelationships was proposed by Arcilla and Gasul, providing a better understanding of cardiac anatomy (10).
Previous studies have relied on echocardiographic, angiocardiographic, or prenatal ultrasound data to evaluate dextrocardia. Although echocardiography remains the first-line modality for assessing these patients, recent advances in CT angiography techniques now allow for a comprehensive analysis of heart structure, particularly vascular anatomy, along with associated extracardiac abnormalities in complex patients. Several studies have emphasized the essential role of cardiothoracic CT angiography in evaluating cardiac malformations, including ventricular morphology, atrioventricular (AV) connections, MAPCAs, great artery relations, and intrathoracic extracardiac anomalies. Many studies have demonstrated that various cardiac anomalies, including AV and ventriculoarterial discordance, malposition of the great arteries, univentricular heart, AV valve abnormalities, and pulmonary atresia, are associated with dextrocardia and often require cardiac surgery (11). Some studies, including one by Yury Rapoport et al., have suggested that preoperative cross-sectional imaging should be performed in the management of dextrocardia due to the high risk of associated anomalies (12).
2. Objectives
To our knowledge, this is the first study to focus on the structural abnormalities of dextrocardia in pediatric patients using CT angiography and the Arcilla and Gasul classification, with the aim of guiding clinicians and surgeons in treatment planning.
3. Methods
The study was approved by the university's ethics committee, and written informed consent was obtained from all participants.
3.1. Patients
In this retrospective cross-sectional study, among the 667 patients referred to the Children’s Medical Center for cardiothoracic CT angiography between January 2010 and March 2019, we enrolled all 48 patients diagnosed with dextrocardia. All patients were under 15 years old. Of these, 12 patients were identified as having dextroposition, and one patient was referred after cardiac surgery; these patients were excluded from the study. Ultimately, 35 cases with a confirmed diagnosis of dextrocardia were considered eligible for inclusion in the study. All patients had previously undergone an echocardiographic exam and were referred for CT angiography after cardiologist assessment to evaluate vascular structures and associated uncertain cardiac anomalies. The primary focus was on identifying major aortopulmonary collateral arteries (MAPCAs), the presence of non-confluent pulmonary arteries, and bronchial tree anatomy in cases of suspected isomerism syndromes, which were challenging to assess with echocardiography.
3.2. CT Scan Protocol
Contrast-enhanced chest CT scan images were obtained in the supine position using a GE 16-slice scanner. The following parameters were used to obtain the images: Tube voltage of 80 - 100 kilo voltage peak (kVp), (80 kVp for children under 2 years and 100 kVp for those older than 2 years), effective current of 60 - 80 mA, pitch of 1.375 - 1.75, matrix size of 512 × 512, and a slice thickness of 0.6 mm (reconstructed slice thickness of 1.25 mm). Limited cuts of the abdomen were also obtained to assess the anatomy of the liver and spleen. The reconstructed images were sent to the picture archiving and communication system (PACS). A total contrast dose of 1.5 mL/kg of a low or iso-osmolar contrast agent was used, with a maximum dose limit of 120 mL, which was not reached in any patient.
3.3. Imaging Interpretation
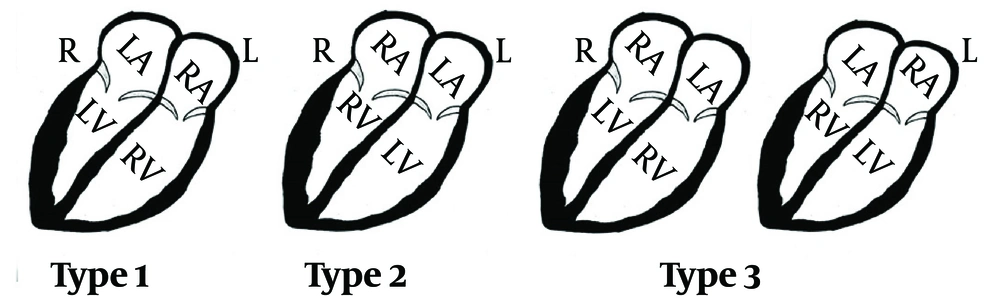
All CT scans were reviewed blindly by a radiologist specialized in cardiovascular imaging. Ten patients with suspicious small septal defects in suboptimal images were recalled and double-checked with echocardiography, with no discrepancies observed between the CT angiography and echocardiographic findings. The CT scan evaluation was conducted across nine major categories: Type of dextrocardia, septal anomaly, atrial anomaly, ventricular anomaly, aortic anomaly, pulmonary artery anomaly, pulmonary vein anomaly, systemic vein anomaly, MAPCAs, and non-cardiac anomalies (including pulmonary, bronchial tree, spleen, liver, thoracic skeletal, and esophagus). The type of dextrocardia was determined based on the Arcilla and Gasul classification (Figure 1).
The classification used in this study includes five major types of dextrocardia: (1) type I, mirror-image dextrocardia, where the anatomic right atrium and right ventricle are situated to the left and anterior; (2) type II, dextroversion complex, characterized by normal chamber relations with an abnormal cardiac axis; (3) Type III, mixed dextrocardia, involving inversion of either the atria or the ventricles alone; (4) Type IV, congenital dextroposition, where the heart is positioned in the midchest with a normal axis; (5) Type V, congenital extrinsic dextroposition, where the heart is abnormally positioned due to external factors.
We excluded cases classified as Type IV and Type V because they do not represent true dextrocardia according to the proposed definition.
In this study, the right atrium was identified as the chamber receiving the inferior vena cava. Due to variations in pulmonary vein anatomy, the left atrium was identified based on its morphology, characterized by a narrow orifice with a long, tubular appendage (13). The right ventricle was identified by the presence of a moderator band, trabeculation from the septum to the free wall, and a muscular outflow tract (the infundibulum). The left ventricle was distinguished by the absence of the infundibulum, a smooth septal surface, and distinct papillary muscles (13, 14).
Bilateral right-sidedness, characterized by bilateral three-lobed lungs with or without asplenia, was considered indicative of right isomerism, while left isomerism was defined by bilateral left-sidedness, characterized by bilateral two-lobed lungs with or without polysplenia. L-looped transposition of the great arteries (L-TGA) was defined as the position of the aortic root being left and anterior to the pulmonary root (15).
3.4. Statistical Analysis
IBM SPSS statistics v.26 was used for statistical analysis. Frequencies and descriptive statistics were calculated for all variables. The distribution of findings among the three types of dextrocardia was compared. To determine the relationship between categorical variables, Pearson's chi-square test was used, with a significance level set at P < 0.05.
4. Results
Thirty-five cases of dextrocardia [18 (51.4%) males and 17 (48.6%) females] were included in this study. The mean age of the patients was 24 months (ranging from 4 days to 14 years). The majority of cases were classified as type 3 (23 cases), followed by type 1 (8 cases) and type 2 (4 cases). The associated cardiac anomalies for each type are summarized in Table 1.
| Associated Anomaly | Type 1 (n = 8) | Type 2 (n = 4) | Type 3 (n = 23) |
|---|---|---|---|
| Septal | - | - | - |
| ASD | 3 (37.5) | 1 (25) | 1 (4.3) |
| VSD | 5 (62.5) | 1 (25) | 1 (4.3) |
| AVSD | 2 (25) | 2 (50) | 22 (95.7) |
| PFO | - | - | - |
| Aortic | - | - | - |
| Right side arch | 3 (37.5) | 1 (25) | 3 (13) |
| CoA | - | 1 (25) | 1 (4.3) |
| Hypoplastic arch | - | 1 (25) | 1 (4.3) |
| Double arch, As, aberrant SA, truncus arteriosus, interrupted arch | - | - | - |
| PDA | 3 (37.5) | - | 10 (43.5) |
| Pulmonary artery | - | - | - |
| PA | 2 (25) | - | 10 (43.5) |
| PS | 2 (25) | 2 (50) | 7 (30.4) |
| Focal stenosis | 2 (25), bilateral | 2 (50), one right and one left side | 2 (6.7), one left and one bilateral |
| MAPCA | - | - | 3 (13) |
| Pulmonary vein | - | - | - |
| TAPVC | - | - | 5 (21.8) |
| Supracordis | - | - | 4 (17.4) |
| Cordis | - | - | 1 (4.3) |
| Infracordis | - | - | 1 (4.3) |
| PAPVC | 2 (25) | - | 2 (6.7) |
| Systemic vein | - | ||
| L-SVC | 4 (50) | - | 7 (30.4) |
| Double SVC | 1 (12.5) | 1 (25) | 6 (26.1) |
| Interrupted SVC/azygous continuation | 2 (25) | 1 (25) | 1 (4.3) |
| Atrial | - | - | - |
| Common atrium | 1 (12.5) | 1 (25) | 7 (30.4) |
| Cor triatriatum, TA, MA | - | - | - |
| Ventricle | - | - | - |
| Hypoplastic | 2 (25) | - | 7 (30.4) |
| RV | - | - | 1 (4.3) |
| LV | 2 (25) | - | 6 (26.1) |
| Single ventricle | - | 1 (25) | 3 (13) |
| Hypertrophy | 2 (25) | - | 2 (6.7) |
| RVH | 1 (12.5) | - | 1 (4.3) |
| LVH | 1 (12.5) | - | 1 (4.3) |
| DORV | 5 (62.5) | 2 (50) | 12 (52.2) |
| L-TGA | 4 (50) | 1 (25) | 20 (87) |
Associated Cardiac Anomalies in 3 Types of Dextrocardia a
Table 2 presents the non-cardiac anomalies observed in each group.
| Associated Anomaly | Type 1 (n = 8) | Type 2 (n = 4) | Type 3 (n = 23) |
|---|---|---|---|
| Right isomerism | 2 (25) | - | 17 (73.9) |
| Left isomerism | 2 (25) | - | 3 (13) |
| Situs inversus | 2 (25) | - | 1 (4.3) |
| Asplenia | 1 (12.5) | - | 8 (34.8) |
| Polysplenia | 2 (25) | 1 (25) | 3 (13) |
| Midline liver | 2 (25) | - | 8 (34.8) |
| Thoracic skeletal | - | - | - |
| Esophagus | - | 1 (25) | - |
Associated Non-cardiac Anomalies in 3 Types of Dextrocardia a
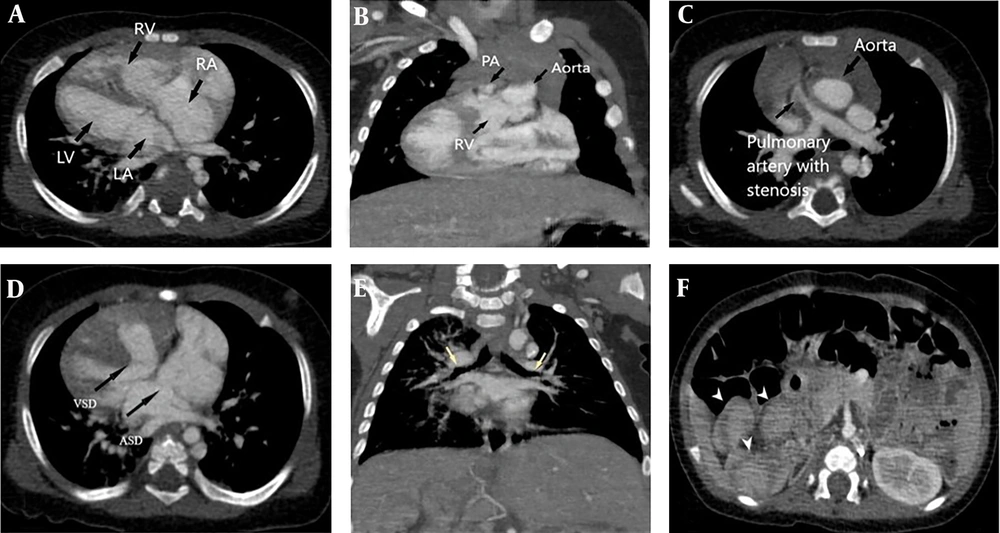
The most common anomalies in type 1 were septal defects (7 cases in total), with ventricular septal defects present in 5 cases. Among these, 3 patients also had a concomitant atrial septal defect (ASD). Six patients exhibited some form of systemic vein anomaly. Double outlet right ventricle (DORV) was also relatively common, occurring in 5 cases. None of these anomalies were significantly more frequent in this type compared to the other two types. There was one patient in type 1 who had no associated anomalies (Figure 2).
Type 1 dextrocardia in a 4-month-old male associated with left isomerism syndrome, double outlet right ventricle (DORV), pulmonary stenosis, left malposition of the great arteries (L-MGA), atrial septal defect (ASD) and VSD. A, correlation of cardiac chambers in type 1 dextrocardia; B, DORV; C, L-MGA and pulmonary stenosis; D, ASD and VSD; E, upper bronchi of both upper lobes (yellow arrows) are hypo-arterial in left isomerism syndrome associated with midline liver; and F, polysplenia (arrowheads).
Type 2 was the least common, with only 4 cases. All patients in this group had some form of septal defect, including 2 with complete atrioventricular septal defects (AVSD), 1 with atrial septal defect (ASD), and 1 with ventricular septal defect (VSD). Two cases had DORV, both of which were associated with AVSD. One of these cases also presented with esophageal atresia, a single ventricle, and L-TGA. No instances of isomerism or situs inversus were observed in this group (Figure 3).
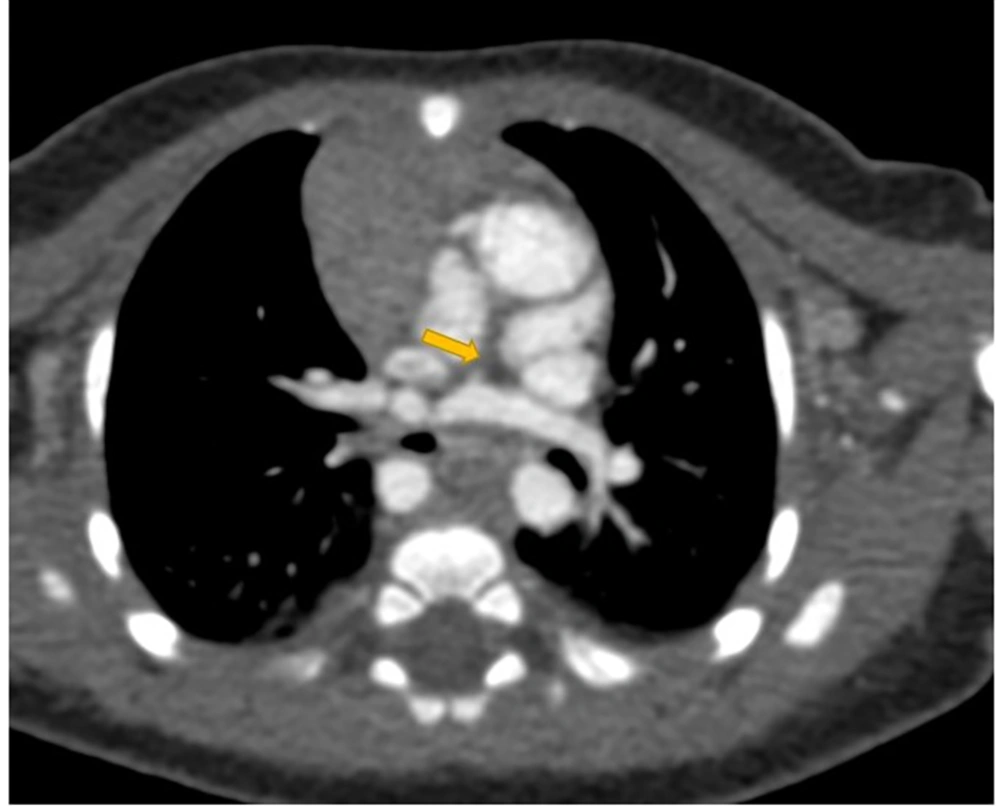
Type 2 dextrocardia in a one-month-old female associated with aortic coarctation and tracheal bronchus. A, correlation of cardiac chambers in type 2 dextrocardia, black arrowhead shows the moderator band in right ventricle (RV); B, solitus major arteries; C, aortic coarctation; D, tracheal bronchus (yellow arrow).
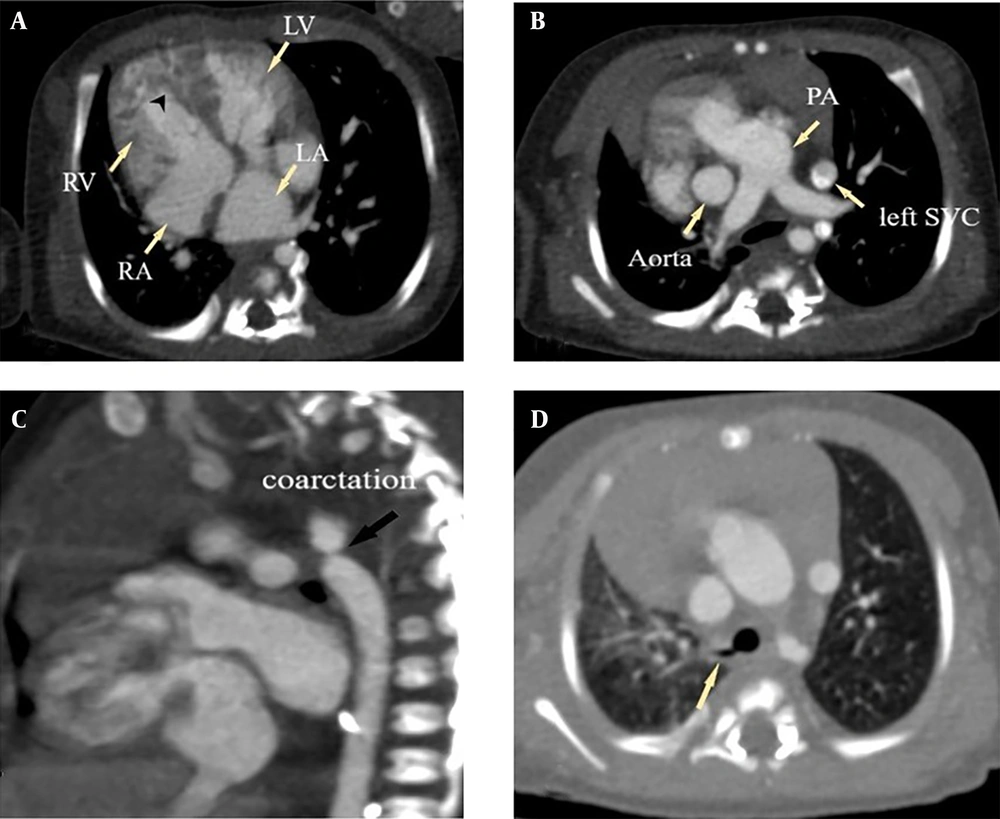
Among the type 3 patients, 22 cases had AVSD, with 19 of these also having concomitant L-TGA. Fourteen patients (61%) had the triad of AVSD, L-TGA, and either pulmonary atresia or stenosis. Systemic vein anomalies were relatively common in this group, occurring in 13 cases (56.5%), all of which had AVSD, and 10 of these also had L-TGA concurrently. Total anomalous pulmonary venous return (TAPVR), MAPCA, and right ventricular hypoplasia were exclusively observed in type 3 patients. Right isomerism was present in 17 cases (73.9%); however, only 6 of these had both asplenia and a midline liver, while 2 had only asplenia and another 2 had only a midline liver (Figure 4).
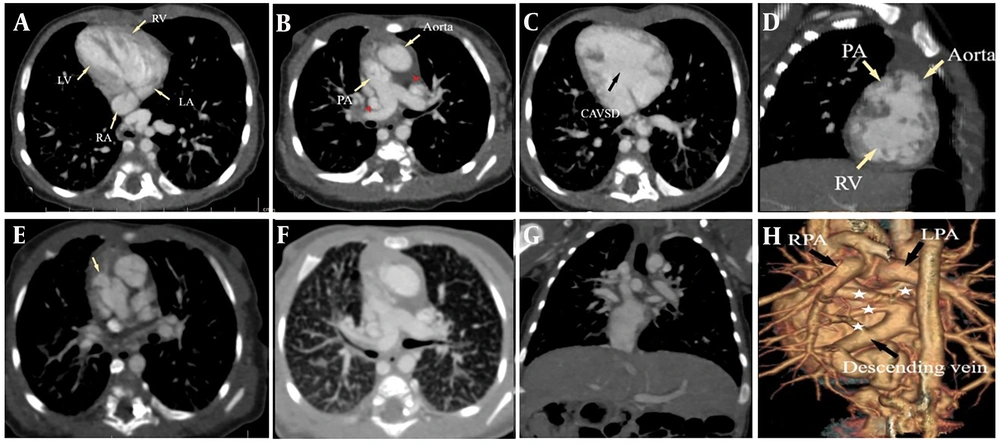
Type 3 dextrocardia in a one-month-old female associated with right isomerism syndrome, CAVSD, double outlet right ventricle (DORV), L-looped transposition of the great arteries (L-TGA), Pulmonary stenosis, infracardiac total anomalous pulmonary venous return (TAPVR), pulmonary edema and double Superior vena cava (SVC). A, correlation of cardiac chambers in type 3 dextrocardia. RA is shown in level of insertion of suprahepatic IVC; B, L-TGA and double SVC (Red arrowheads); C, compete atrioventricular septal defects (AVSD); D, DORV; E, subvalvular pulmonary stenosis; F, epiarterial upper lobe bronchi of both lungs (right isomerism) and also interlobular septal thickening due to pulmonary edema; G, midline liver; H, reconstruction of heart in posterior view showing infracardiac TAPVR (white stars show pulmonary veins).
As shown in Table 1, the prevalence of certain anomalies is notably higher in the type 3 group. When comparing anomalies between type 3 and non-type 3 groups, we found that the following anomalies were significantly more common in type 3 patients: L-TGA (X² = 7.926, P = .005), right isomerism (X² = 10.414, P = 0.001), and AVSD (X² = 16.032, P < 0.001). These three anomalies were concurrently present in 15 patients (65.2%) within the type 3 group. Although pulmonary artery abnormalities did not show a statistically significant difference in type 3 patients, they occurred concomitantly with the aforementioned anomalies in 12 cases (52.2%). This high prevalence of concurrent anomalies is particularly evident in type 3 dextrocardia.
5. Discussion
The purpose of this study was to investigate the associated anomalies found in dextrocardia patients during cardiac CT angiography, focusing on the different morphologic types of dextrocardia, in order to provide clinical guidance for both cardiologists and surgeons. To our knowledge, this is the first study that specifically examines the associated findings in subtypes of dextrocardia using cardiac CT angiography.
Despite various definitions and classifications for dextrocardia, the most widely accepted definition describes it as a heart with a base-apex axis oriented toward the right side of the body (5). However, the orientation of the heart alone is not the primary concern; it is the associated cardiac and non-cardiac abnormalities that could be lethal if left untreated (6, 7, 16). We used Arcilla and Gasul's typing system to investigate the structural abnormalities of the heart (10). Based on this classification, we found that the most common type was type 3, with a prevalence of 65.7%. However, this does not necessarily indicate that type 3 is the most common form of dextrocardia overall. Since the prevalence of associated cardiac abnormalities is higher in type 3, and these cases require more complex surgical and medical treatments, they are more likely to be referred to our tertiary center than the other two types. In another study using the same classification, researchers investigated dextrocardia and associated anomalies in prenatal cases and found that of the 22 cases of fetal dextrocardia, 12 were type 2, 6 were type 1, and 4 were type 3 (9).
We found that the prevalence of L-TGA, AVSD, and right isomerism is significantly higher in type 3 patients, with these three anomalies occurring together in 65% of cases. Additionally, 52% of type 3 patients had an additional pulmonary artery abnormality (Figure 5).
Lev et al. discussed the pathological anatomy and embryological aspects of dextrocardia and also explained hypotheses regarding associated anomalies. They suggested that pivotal atria could be accompanied by inverted transposition and ventricular septal defects, often associated with a straddling aorta and pulmonary stenosis. Additionally, the association between splenic abnormalities, such as asplenia, and endocardial cushion defects with trunco-conal malformations, such as inverted transposition, has been demonstrated in previous studies. However, the basic factors responsible for the association between the pivotal atria and the L-bulbo-ventricular loop remain unexplained (17, 18).
This is of significant clinical and diagnostic importance, underscoring the need for radiologists and cardiologists to carefully evaluate all these anomalies. The prevalence of these anomalies in dextrocardia patients has been reported variably, depending on the diagnostic method and classification system used (7-9, 16, 17, 19, 20). Although there is currently limited evidence, the possibility of concomitant anomalies in type 3 dextrocardia should be considered. More studies focusing on these anomalies are needed to validate this concern.
In our study, eight cases of left ventricle hypoplasia were observed, with six cases in type 3 and two cases in type 1. All these cases had DORV, and AVSD and right isomerism were present in seven of them. Additionally, six cases (all in type 3) also had L-TGA. Consistent with our findings, some prenatal studies have reported cases of left ventricle hypoplasia (9, 21, 22). In one of these studies, of the three cases, two had concomitant DORV and one had AVSD (9). However, in contrast to our findings, some studies reported no cases of left ventricular hypoplasia (2, 4, 7, 16, 17, 19).
Previous studies using echocardiography have reported cases of valvular abnormalities in dextrocardia (7, 8). However, we did not identify any such cases in our study. This discrepancy could be attributed to the limitations of cardiac CT angiography in evaluating valvular disease (21). In the study by Epcacan et al., concomitant cardiac malformations were evaluated using echocardiography and compared across three subgroups: Situs solitus, situs ambiguous, and situs inversus dextrocardia. They found that situs solitus was the most common subgroup with additional cardiac anomalies, followed by situs ambiguous and situs inversus. However, this study did not evaluate the subtypes of dextrocardia based on the Arcilla and Gasul classification (23). Bohun et al. found that the most complex cardiac malformations were associated with isomerism, while ventricular septal defects were the most common type of cardiac malformation in situs solitus and situs inversus, likely due to their higher inherent prevalence. Situs inversus showed a lower association with cardiac malformations compared to situs solitus and isomerism (6). Rapoport et al. suggested that preoperative assessment, including cross-sectional imaging such as CT scans and MRI in addition to echocardiography, should be performed in patients with dextrocardia, particularly those with situs solitus, for optimal surgical planning due to the complex cardiac and extracardiac anomalies associated with dextrocardia (12). Both cardiothoracic surgeons and anesthesiologists face specific challenges with these patients, making detailed preoperative assessment critically important.
To our knowledge, no other study has focused on this classification system of dextrocardia and the associated anomalies in the same manner as ours.
This study had some limitations. First, the sample size was not sufficient to conduct more detailed analytical studies. Second, since our hospital is a referral center for pediatric cardiac abnormalities, the prevalence of the disease in our study may not be generalizable. Third, some patients underwent cardiac surgery based solely on echocardiographic results without preoperative cardiac CT angiography, which excluded them from our study. Finally, due to concerns about radiation dose, we were unable to obtain full abdominal images to optimally evaluate associated non-cardiac abnormalities.
Cross-sectional imaging is usually necessary for analyzing ventricular morphology, AV connections, and the relationships of the great arteries. Echocardiographic examination in patients with dextrocardia can be more challenging due to technical issues (13). Cardiac CT angiography and MRI serve as adjuncts to echocardiography, which remains the primary modality, with each imaging technique having its own limitations and advantages. The comprehensive assessment of the heart and the evaluation of extracardiac anomalies are the main advantages of cardiac CT and MRI over echocardiography. Cardiac CT angiography has gained several advantages in recent years. Compared to echocardiography, it provides a more global assessment of cardiovascular anatomy, particularly for MAPCAs, pulmonary arteries and veins, the thoracic aorta, and systemic veins. Additionally, it provides valuable information about adjacent structures, including skeletal, pulmonary, airway, esophageal, and upper abdominal organs. Cardiac CT angiography is less operator-dependent and faster (22). Techniques such as ECG-gated CT scanning with ECG-pulsing, low tube voltage, and automatic tube current modulation can reduce radiation dose while providing high-quality images.
Cardiac MRI offers essential quantitative and qualitative data, including ventricular function and volumetric assessment, 3D and 4D imaging, evaluation of myocardial inflammation, pressure measurement, and does not involve radiation exposure (24, 25). Compared to MRI, cardiac CT requires less sedation, is faster, and has no contraindications related to metal devices. Additionally, CT allows better observation of the child during the examination, making it more suitable for ill patients. Although cardiac catheterization provides valuable physiological data, it has several disadvantages, including a higher radiation dose, longer sedation time, and less detailed anatomical information (22, 26). Despite the advantages of cardiac CT angiography, such as detailed anatomical visualization, there are concerns about its limitations, including the lack of functional data, radiation exposure, challenges in evaluating valvular abnormalities, and the potential for adverse reactions to contrast agents. Today, echocardiography remains the primary modality for assessing these patients due to its widespread availability, absence of ionizing radiation, and low cost. However, it may lack the ability to provide detailed insights into certain cardiac and extracardiac anomalies, especially with 3D reconstruction, which cannot be achieved with echocardiography (27). Therefore, while CT angiography can serve as a supplementary modality, its use, particularly in pediatric patients, should be carefully justified to identify doubtful anomalies and complex structures while minimizing the risks associated with radiation exposure (28).
5.1. Conclusions
our study revealed that in type 3 dextrocardia, special attention should be given to the following anomalies: AVSD, L-TGA, right isomerism, pulmonary artery anomalies (including atresia or stenosis), and DORV. There may be a significant correlation among these anomalies in type 3 dextrocardia, and recognizing this potential correlation as a unique and specific cardiac anomaly could assist cardiologists, radiologists, and surgeons in better managing these cases. Based on the results of this study, and with support from future studies, cardiothoracic CT angiography could be considered as an adjunct to echocardiography in the diagnostic workup, after a careful cost-benefit analysis and assessment of indications, particularly when approaching patients with type 3 dextrocardia and other complex cardiac anomalies. The detailed information provided by CT angiography could help in developing appropriate surgical treatment strategies, which may justify the use of this modality.
Additionally, diagnosing asplenia in these patients can alert pediatricians to implement appropriate vaccination protocols and provide specialized patient care. Future studies should further explore these issues. We emphasize that although cardiac CT angiography is useful in detecting cardiac anomalies, it should be reserved for evaluating patients with uncertain or undetectable cardiac or vascular anomalies on echocardiography.