1. Background
Very preterm delivery is often associated with adverse perinatal and neonatal outcomes (1). The first hours of a premature infant's life are crucial for developing the risks of hypoxic-ischemic encephalopathy (HIE) due to hemodynamic instability and respiratory distress syndrome (RDS) (2). These consequences can lead to short and long-term adverse neurodevelopmental outcomes (3, 4).
Compared to their full-term counterparts, premature neonates require aggressive external surfactant administration and other respiratory support to reduce the risk of RDS, air leakage, bronchopulmonary dysplasia, and death (5). However, surfactant treatment may have some adverse effects. It has been shown that early surfactant therapy for intubated preterm infants with RDS can lead to increased muscular tone (6). Surfactant administration can also increase the duration of maximal blood flow in the internal carotid artery by up to 100% above baseline in some patients. Besides the acute effects of surfactant treatment on hemodynamic status, changes in cerebral perfusion may occur due to rapid changes in the partial pressures of intracerebral tissue gases (PO2 and PCO2), affecting cerebral vascular resistance. These effects may be detrimental because the increased risk of intraventricular hemorrhage in premature infants can occur due to the loss of autoregulation of cerebral vessels resulting from decreased cerebral perfusion pressure (7, 8). Therefore, monitoring regional cerebral oxygen saturation (rScO2) in premature infants is essential to assess cerebral oxygenation.
Pulse oximetry (PO), considered the gold standard method, is typically used to estimate rScO2 and heart rate during neonatal revival (9). These clinical parameters provide specialist medical practitioners with accurate diagnostic information and guide interventions to support the neonatal resuscitation process (10). It has been established that sensitive assessment of rScO2 is crucial to regulate inspired oxygen concentrations to prevent hypo/hyperoxia (11, 12).
2. Objectives
In recent times, near-infrared spectroscopy (NIRS) has been considered a useful device for the non-invasive, continuous, real-time monitoring of absolute regional tissue oxygen saturation (rStO2) (12, 13). Previous studies have shown associations between ScO2 with superior vena cava blood flow and left ventricular cardiac activity in preterm neonates (14-16). Monitoring cerebral oxygenation by NIRS is presumed to be a highly efficient diagnostic tool to assess low cerebral oxygen delivery in preterm neonates during neonatal resuscitation. Therefore, this study aimed to investigate the relationship between oxygen saturation measured by PO vs NIRS following surfactant therapy in very low-birth-weight (VLBW) neonates.
3. Methods
This observational, prospective pilot study was designed to investigate VLBW (<1500 g) preterm neonates admitted to the neonatal intensive care units (NICUs) of Mofid and Mahdieh hospitals affiliated with Shahid Beheshti University of Medical Sciences in Tehran, Iran, in 2018. The parents of the participants were informed about the study's objectives, and the confidentiality and anonymity of collected data were ensured. Written informed consent was obtained from the parents before their neonates' participation.
Inclusion criteria were premature birth, birth weight less than 1500 g, receiving surfactant administration, and the possibility of using cerebral NIRS in the first hours of life. Neonates without parental written informed consent were excluded from the study.
Surfactant was administered through an endotracheal tube to neonates who met the inclusion criteria and had moderate to severe RDS based on the INSURE (INtubation-SURfactant-Extubation) approach (17). Peripheral and cerebral oxygen saturation levels were continuously monitored using a Masimo SET Radical pulse oximeter (Masimo Corp, Irvine, CA, USA) before, during, and 5 and 10 min after the surfactant injection (SI). A PO sensor (which included 2 light-emitting diodes for red light and infrared) and a photodiode detector were placed on the right wrist of each VLBW neonate and connected to the monitor. Additionally, 2 pediatric cerebral sensors of the near-infrared spectrometer (INVOS 5100 c; Somanetics Corp, Troy, MI, USA) were bilaterally attached to the neonates' forehead area to monitor rScO2 levels during the first 3 h after birth.
Considering the possibility of oxygen fluctuations during surfactant administration changes in oxygen saturation rates were assessed and simultaneously recorded using both PO and NIRS. Mean arterial blood pressure (MABP) values were also recorded alongside other measurements before, during, and 5 and 10 min after SI.
All demographic data related to the participants and their mothers, including the mother's gravidity, type of delivery, history of chorioamnionitis or preeclampsia, antenatal corticosteroid administration, neonate's gender, birth weight, gestational age, Apgar scores at 1 and 5 min, history of RDS, mechanical ventilation requirement, and frequency of surfactant administration, were collected and recorded.
The objective of the present study was to determine any difference between the saturation measured by NIRS vs PO.
3.1. Statistical Analysis
Data analysis was performed using SPSS version 16 (SPSS Inc, Chicago, IL, USA). In the descriptive analysis, normally distributed continuous data were presented as mean ± SD, while abnormally distributed data were represented as median with an interquartile range.
The repeated measures analysis of variance (ANOVA) test was used to show the changes in variables recorded by PO or NIRS at each evaluation time. The independent t-test was used to compare continuous variables between the 2 groups. Spearman's correlation was calculated to assess whether a non-linear/linear relationship existed between the studied variables. P-values less than 0.05 were considered significant.
4. Results
Of the 22 VLBW neonates, 2 patients were excluded (one due to pneumothorax and the other due to the need for resuscitation). Nine (45%) mothers were primiparous. The number of parity in 5 (25%), 4 (20%), and 2 (10%) mothers were 2, 3, and 4, respectively. Antenatal corticosteroid therapy was reported in 13 mothers (65%) of the neonates. The mean birth weight of the neonates was 1063 g (minimum: 600; maximum: 1420), and their mean gestational age was 28.44 weeks (minimum: 24; maximum: 34). Most neonates were delivered by cesarean section. Among all cases, 75% received surfactant once, while the rest received it twice during the study period. The median Apgar score improved from 6.50 at 1 min to 8.00 at 5 min. Table 1 shows the demographic characteristics of the included neonates.
| Variables | Values |
|---|---|
| Gender, No. (%) | |
| Female | 12 (60) |
| Male | 8 (40) |
| Type of delivery, No. (%) | |
| Cesarean | 19 (95) |
| Vaginal | 1 (5) |
| Birth weight, g, mean ± SD [min-max] | 1063 ± 246 [600 - 1420] |
| Gestational age, w, mean ± SD [min-max] | 28.44 ± 2.57 [24 - 34] |
| Apgar score at 1 min, median (mean ± SD) [min-max] | 6.50 (6.05 ± 2.57) [1.0 - 9.0] |
| Apgar score at 5 min, median (mean ± SD) [min-max] | 8.00 (7.94 ± 1.79) [4.0 - 10.0] |
| Surfactant administration, No. (%) | |
| Once | 15 (57) |
| Twice | 5 (25) |
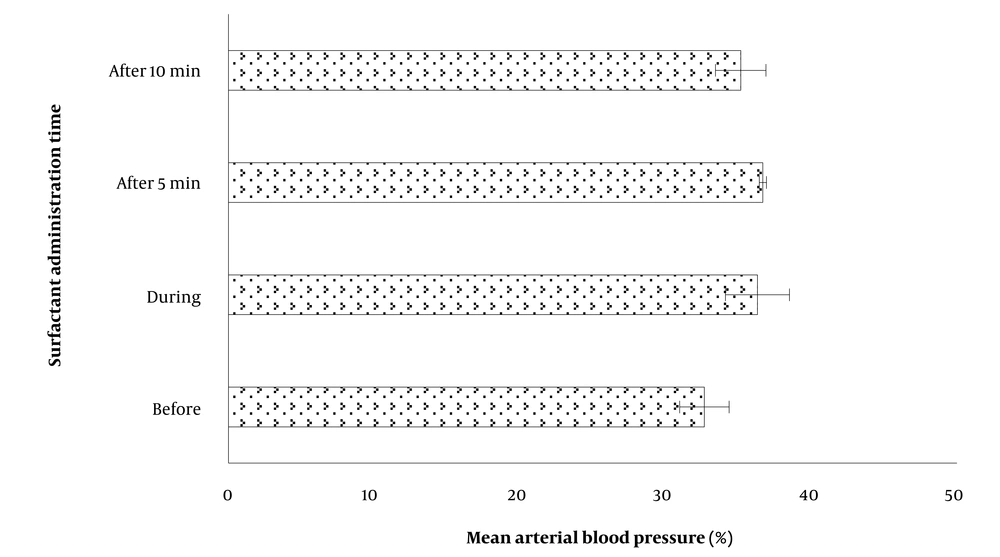
| MABP, mean ± SD [min-max] | |
| Before SI | 32.66 ± 7.60 [18 - 46] |
| During SI | 36.31 ± 9.82 [24 - 63] |
| 5 min after SI | 36.68 ± 1.08 [18 - 71] |
| 10 min after SI | 35.16 ± 7.76 [23 - 52] |
Abbreviations: MABP, mean arterial blood pressure; SI, surfactant injection.
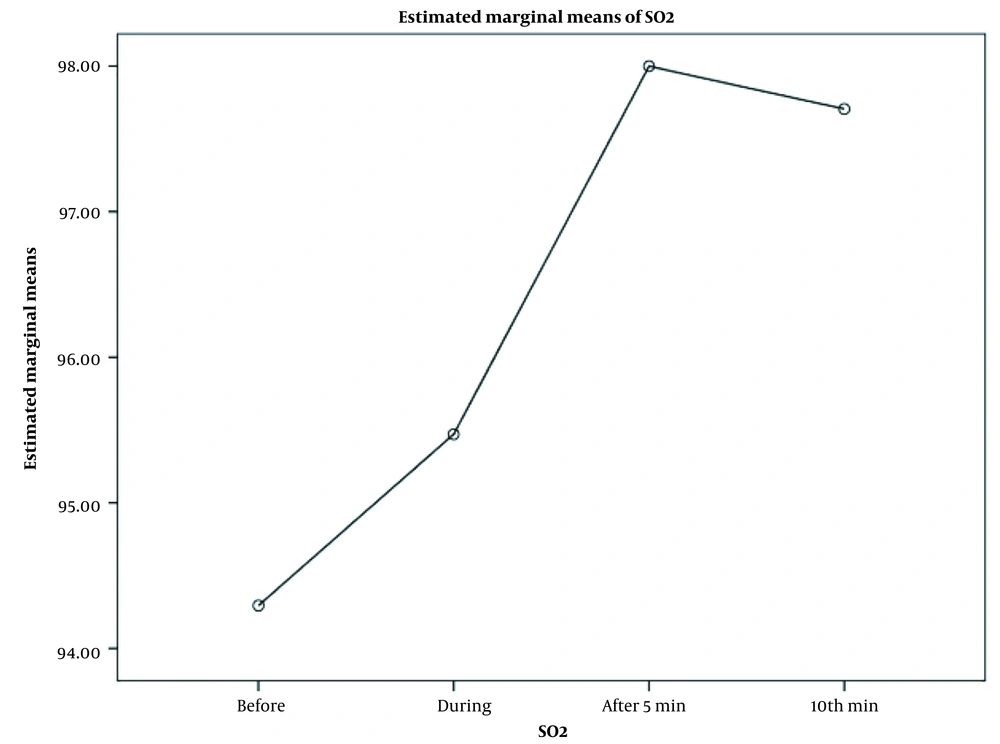
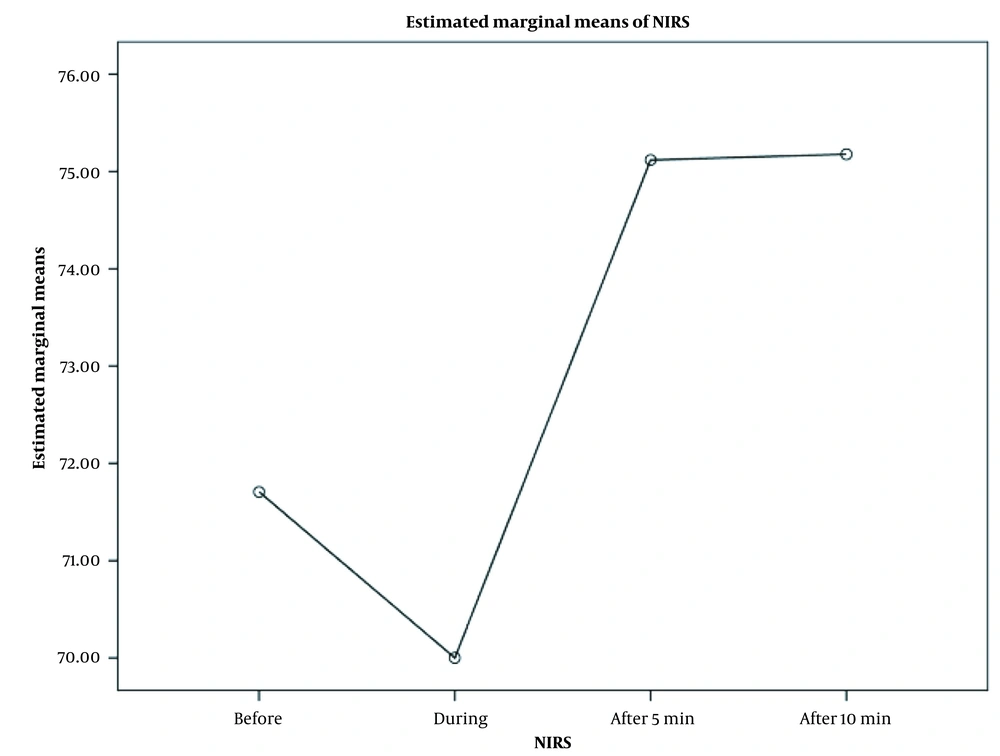
As shown in Figures 1 and 2, there were significant changes in mean oxygen saturation (recorded by PO; P = 0.002) and mean rScO2 (recorded by NIRS; P = 0.007) at each evaluation time (before and after SI). The figures also show the highest oxygen saturation and rScO2 values during 5 min after SI.
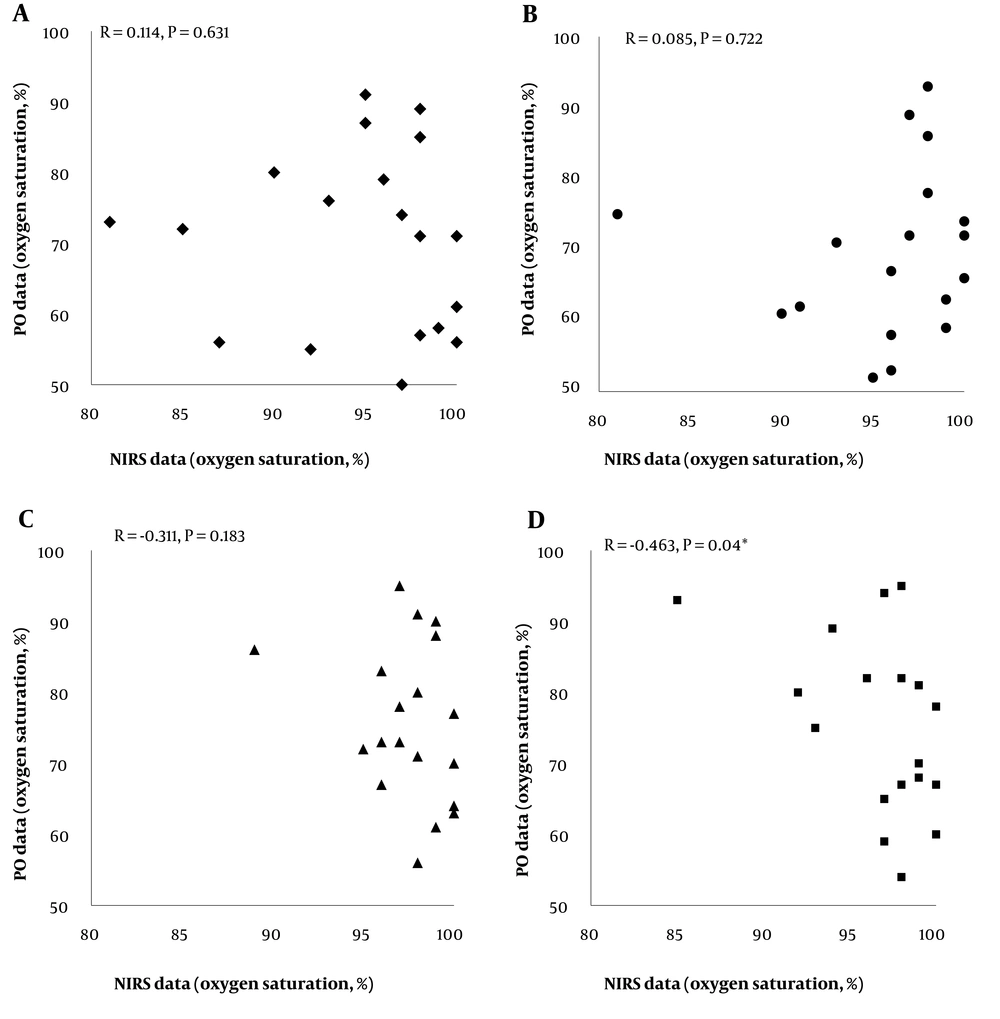
Based on the results, there were no significant differences between arterial and tissue oxygen saturation values assessed by the 2 devices (PO and NIRS) before (P = 0.631), during (P = 0.722), and at 5 min after SI (P = 0.783). However, a significant inverse correlation was detected between the oxygen saturation levels measured by PO and NIRS at 10 min after SI (P = 0.04, r = -0.463). Detailed data are shown in Figure 3.
The Spearman's correlation results between the oxygen saturation levels measured by pulse oximetry (PO) and near-infrared spectroscopy (NIRS) devices at different surfactant administration times; there were no significant differences between arterial and tissue oxygen saturation values assessed by the 2 devices before (A), during (B), and at 5 min after SI (C). However, a significant inverse correlation was detected between the oxygen saturation levels measured by PO and NIRS at 10 min after SI (D). Abbreviations: NIRS, near-infrared spectroscopy; PO, pulse oximetry.
Regarding the associations between MABP and rScO2 levels detected by the INSURE approach at different times of SI, there was an insignificant gradual increase (3.65%) in MABP immediately during surfactant therapy (Table 1 and Figure 4). Nonetheless, there were no significant associations between MABP and NIRS- rScO2 levels before (P = 0.971, r = -0.009), during (P = 0.194, r = -0.312), at 5 min (P = 0.438, r = -0.189), and at 10 min (P = 0.896, r = -0.033) after SI.
5. Discussion
The results of the present study (which showed no differences between oxygen saturation [recorded by PO] and rScO2 [recorded by NIRS] rates before, during, and after SI) suggest that using PO in NICUs to determine cerebral oxygenation, autoregulation, and hypoxia is reasonable and cost-effective. A PO device is commonly used to measure surfactant efficacy in the lungs, and NIRS has been recently suggested as a rapid and non-invasive technique for a similar effectiveness assessment (18-20). In this study, rScO2 levels measured by NIRS were compared to PO values at 4 different times: Before, during, and 5 and 10 min after SI. To our knowledge, this study is the first report from Iran in which NIRS was implemented to monitor rScO2 and MABP in premature VLBW neonates who were treated with surfactant.
The results of the present study showed that there were no significant differences between oxygen saturation and rScO2 rates recorded by the 2 devices before, during, and 5 min after SI. This finding suggests that NIRS, similar to PO, can assess rScO2 levels before, during, and after SI. The NIRS device is non-invasive and harmless, and after economic evaluations, it could be implemented as an alternative to PO in NICUs to determine cerebral oxygen levels, autoregulation, and hypoxia. Furthermore, the integration of NIRS with electroencephalography (EEG), ultrasound, or other advanced imaging systems can provide comprehensive data on tissue health through multimodal monitoring.
Consistent with our study, several studies have used NIRS as a useful diagnostic tool to assess rScO2 levels among preterm neonates. Li et al. used NIRS technology to evaluate rScO2 levels in 44 preterm newborns. Participants with RDS and surfactant administration were divided into 2 groups: Less invasive surfactant administration (LISA) and INSURE groups. The results showed a significant difference in rScO2 levels between the 2 groups during and after SI. They concluded that the use of surfactant might transiently affect cerebral autoregulation (21).
Underwood et al. also used NIRS to evaluate rScO2 levels in the lungs, brain, skeletal muscle, and kidneys of extremely low-birth-weight (ELBW) neonates during the first 12 h after birth. They concluded that NIRS could be a useful tool in identifying ELBW neonates who would likely benefit from early echocardiography and subsequent intervention to close the patent ductus arteriosus (22).
Dix et al. simultaneously used 2 sensors in 3 NIRS modules to monitor rScO2 in the left and right frontoparietal lobes of 55 preterm neonates. They found a relatively high association between all the sensors used in the 3 NIRS devices. Consequently, they suggested that this tool could be used for the diagnosis and prevention of neonatal hypoxic injury (23).
Schat et al. used NIRS to compare the levels of rScO2 at 2 abdominal locations of preterm infants with suspected necrotizing enterocolitis. While there was a weak correlation between the rScO2 levels in the liver and infra-umbilical regions, a statistically significant difference between the levels of rScO2 in the 2 tissues indicated that the NIRS method could be used to measure the abdominal rScO2 (24).
The results of the present study also showed a statistically significant and inverse correlation between oxygen saturation levels recorded by PO and NIRS devices at 10 min after SI. It seems that by increasing oxygen saturation (recorded by PO), NIRS showed lower levels of rScO2.
Our results also showed no significant associations between MABP and NIRS- rScO2 values assessed by NIRS before, during, and after surfactant therapy. However, an insignificant gradual increase in MABP was observed immediately during surfactant therapy. This finding may be related to cerebral autoregulation, which was marginally reduced with surfactant delivery.
Vesoulis et al. highlighted the simultaneous monitoring of rScO2 and arterial blood pressure using NIRS. They provided valuable data on cerebral autoregulation at a given systemic blood pressure. This concurrent measurement showed cerebral autoregulation when a change in blood pressure was not related to variations in cerebral oxygenation. Conversely, an instant effect on cerebral oxygenation with changing blood pressure indicated a lack of cerebral autoregulation (25).
In contrast, other studies by Verhagen et al. and Pfurtscheller et al. found a direct association between rScO2 and MABP levels monitored by NIRS (26, 27). Li et al. analyzed the levels of rScO2 and MABP in preterm infants with RDS treated by LISA or INSURE at different times before and after surfactant administration. They reported no significant differences in the levels of rScO2 and MABP between the 2 groups before surfactant administration. However, these indicators showed a significant difference during and after surfactant therapy (21).
Regarding participants' characteristics, our results indicated that 65% of mothers had received antenatal corticosteroid therapy to enhance lung maturity in their infants. Almost all neonates in our study were delivered via cesarean section. A comparative analysis revealed that a higher percentage of Chinese mothers received antenatal corticosteroids during pregnancy (about 70%), and only 34% of preterm Chinese neonates were delivered via cesarean section (21). This significant difference in the rates of cesarean delivery may be attributed to factors such as premature birth, obstetrical history, or specific protocols and guidelines for childbirth procedures.
The mean Apgar score among our participants at 1 min was low (6.05), which could be associated with prematurity or the frequency of maternal antenatal corticosteroid therapy. This value was very close to the findings of Li et al., who reported an Apgar score of 7.0 for preterm infants at 1 min (21). Additionally, our study showed that the means of gestational age and birth weight among our participants were 28 weeks and 1063 g, respectively. When comparing these values with other studies, Verhagen et al. reported a very similar median gestational age (29 weeks), although the mean birth weight was higher than that of our subjects (1245 g vs 1063 g). The authors suggested that NIRS monitoring may not be applicable to very young and critically ill neonates (26).
The present study showed that NIRS, similar to PO, could effectively assess rScO2 levels before, during, and after the SI. Additionally, NIRS proved to be a successful method to measure MABP levels. Moreover, our findings showed no significant correlations between rScO2 and MABP values measured by NIRS at various time points during surfactant administration. To enhance the accuracy of rScO2 and MABP assessment using NIRS, optimizing the parameters involved in the monitoring process is recommended. Combining optimized NIRS conditions with surfactant administration may improve within-infant variation by decreasing pulmonary vascular resistance and increasing MABP.
Furthermore, integrating NIRS with other advanced imaging systems (such as EEG or ultrasound) could provide comprehensive data on tissue health through multimodal monitoring. However, it is important to note that the potentially high cost of NIRS monitoring probes may limit their widespread use (28). Given that our study found no significant differences between recorded oxygen saturation and rScO2 rates, using PO in NICUs to assess cerebral oxygenation, autoregulation, and hypoxia remains a reasonable and cost-effective alternative device.
5.1. Limitations
This study has several limitations. First, it was a pilot study with a small population of premature infants weighing less than 1500 g. This limitation could potentially reduce the study's ability to detect differences in the assessed relationships, make adjustments for major confounders, and draw causal inferences. Additionally, the generalizability of the results may be reduced due to the small sample size and minor variations in sensor repositioning during the study.
Another limitation to consider is the potentially high cost associated with NIRS monitoring probes. Moreover, there is a range of commercially available NIRS devices with varying sensitivity and specificity levels, making differences in rScO2 and MABP data obtained. Therefore, future studies with larger sample sizes are needed to comprehensively assess the potential benefits of using NIRS as an adjunctive tool to reduce brain damage in preterm newborns during surfactant administration.
5.2. Conclusions
There were no significant differences between the rates of oxygen saturation (recorded by PO) and rScO2 (recorded by NIRS) before, during, and after SI. Consequently, using PO in NICUs to assess cerebral oxygenation, autoregulation, and hypoxia appears to be a reasonable and cost-effective approach. However, further multicenter studies are needed to confirm the practical advantages and cost-effectiveness of NIRS as an emerging monitoring system.