1. Introduction
Graves' disease (GD), the most common cause of hyperthyroidism, is responsible for 10 - 15% of thyroid disorders in the pediatric population, with a greater prevalence in female patients (1). Recently, an increase in the incidence of autoimmune hyperthyroidism in younger age groups has come to attention (2). The generation of autoantibodies against the thyroid-stimulating hormone (TSH) receptor is the main process involved in the pathogenesis of GD (1). Although the triggering mechanism of autoimmune responses remains unclear, the interaction of environmental factors with genetic predisposition could be influential (3). The diagnosis is made based on the presence of tachycardia and goiter, along with elevated T3 and/or T4 levels and suppressed TSH (1).
The therapeutic strategies for GD primarily include antithyroid medications, radioactive iodine-131 (RAI) therapy, and surgery. However, the severity of symptoms, the size of the goiter, and the presence of comorbidities can impact the selection of treatment type (1). Antithyroid medications, especially methimazole, are the preferred treatment for the majority of pediatric patients (4). Methimazole, a drug that inhibits thyroid peroxidase function, is typically prescribed at a dose of 0.15 - 0.5 mg/kg per day (4). Methimazole is associated with minor side effects such as cutaneous rashes, gastrointestinal upset, and arthralgia, which are more common. Major side effects include agranulocytosis, vasculitis, and hepatotoxicity, which are rare but more dangerous (5). The most hazardous complication of methimazole is agranulocytosis, with a reported death rate of 2% (6). Here, we aim to describe an adolescent female with GD who developed agranulocytosis after methimazole administration and discuss her clinical management.
2. Case Presentation
A 13-year-old female with a positive family history of GD and a sibling with vitiligo initially presented with severe sweating, palpitations, and goiter six months prior to her first visit. Initial laboratory testing confirmed GD with suppressed TSH at 0.05 µIU/L (reference range: 0.39 - 4.3 µIU/L, by immunoassay), and elevated T4 and T3 levels at 17.5 µg/dL (reference range: 5.1 - 12.5 µg/dL, by immunoassay) and 7.6 ng/mL (reference range: 0.846 - 2.02 µg/dL, by immunoassay), respectively. Thyroid scintigraphy (Tc-99m) showed a diffuse hyperfunctioning goiter. She was diagnosed with GD and started on methimazole at an initial dose of 10 mg (0.2 mg/kg) daily and propranolol for symptomatic relief.
As optimal thyroid function control was not achieved after two months, the methimazole dose was increased to 20 mg (0.4 mg/kg) daily. She continued on this dose for over four months until she developed a high-grade fever (40°C), sore throat, palpitations, fatigue, myalgia, and diarrhea. Physical examination revealed tachycardia, lymphadenopathy, and a severely erythematous throat. She was admitted to the hospital as her blood testing revealed an absolute neutrophil count (ANC) of less than 500 cells/μL, leading to the immediate discontinuation of methimazole. Table 1 presents her laboratory results at the time of admission.
| Laboratory Range | Parameter Value | Normal |
|---|---|---|
| WBC | 500 | 3.5 - 12. 109/L |
| HB | 11.1 | 13.0 - 17.0 g/L |
| PLT × 1000 | 283 | 150 - 450. 109/L |
| ESR | 101 | |
| CRP | 335 | |
| TSH | 0.05 | mIU/L (0.39 - 4.3) |
| FT3 | 7.3 | ng/mL (0.846 - 2.02) |
| FT4 | 50.4 | µg/dL (5.1 - 12.5) |
Chest CT scan, echocardiography, and abdominopelvic ultrasound showed no pathological findings. The most probable diagnosis was methimazole-induced agranulocytosis. Other causes of neutropenia based on the patient's age and sex, such as systemic lupus erythematosus, malaria, tuberculosis, folate, and vitamin B12 deficiency, were investigated through focused physical examinations and related diagnostic tests. The results did not suggest any condition other than methimazole-induced agranulocytosis, so treatment was started accordingly.
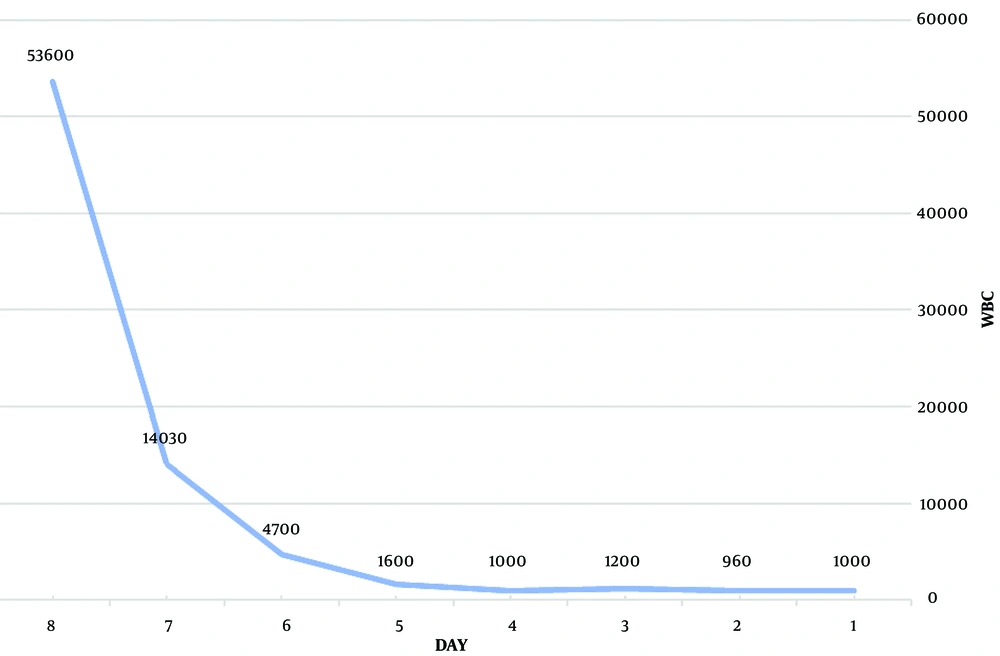
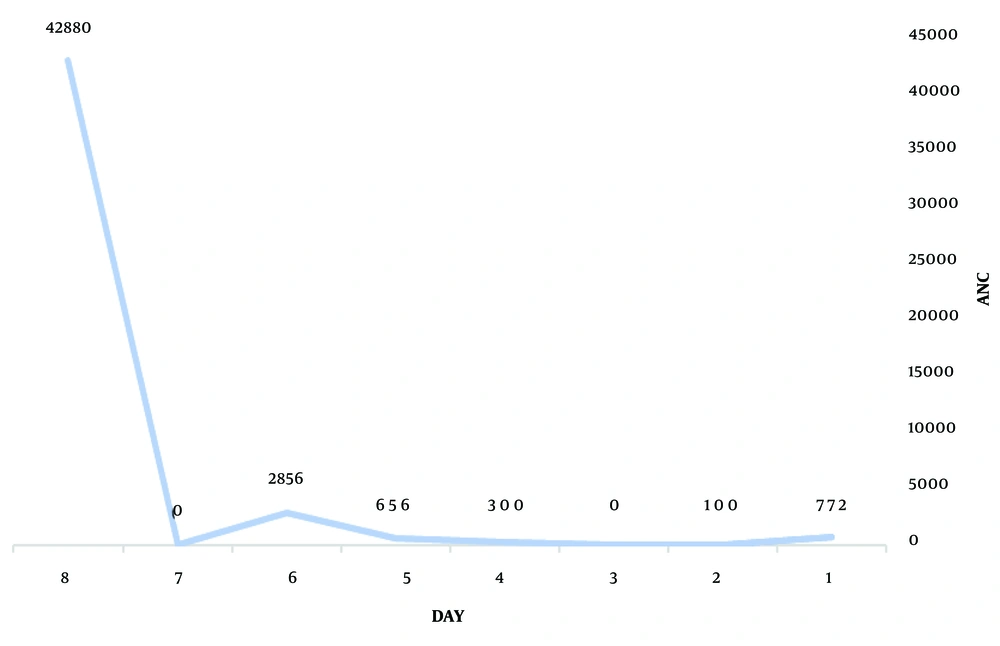
Medications included filgrastim (granulocyte colony-stimulating factor) 5 µg/kg/day by subcutaneous injection, cholestyramine 12 grams daily (260 mg/kg/day in 3 divided doses), propranolol 40 mg (1 mg/kg) daily, lithium 900 mg daily (20 mg/kg/day in 3 divided doses), super saturated potassium iodide (SSKI) 2 drops every 12 hours, and broad-spectrum antibiotics. Diarrhea stopped on the second day of admission, the sore throat resolved on the third day, and the fever subsided on the fourth day. The ANC gradually increased with the mentioned treatments. The details of the patient's laboratory testing during admission and after discharge are demonstrated in Table 2. On the fifth day of admission, the ANC recovered (Figures 1 and 2), and the patient was discharged after eight days.
| Variables | WBC | ANC | HB | PLT × 1000 | FEVER | GCSF | ESR | CRP | MCV | AST/ ALT ALK | BIL T/D | TSH FT3/FT4 | TT3/TT4 T3RU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First day of hospitalization | 1000 (3.5 - 12) 109/L) | 772 | 11.1 0 - 14.72 g/L) | 283 (150 - 450) 109/L | + | 104 | 168 | 73.8 (80 to 96 FI) | 10/5 (8 to 48 U/L) 246(130 - 340 U/L) | 1/0.2 | 0.1 a 7.3 b/50 c | ||
| Second day of hospitalization | 960 | 100 | 10 | 254 | + | 300 | 69 | 176 | 75.19 | ||||
| Third day of hospitalization | 1200 | 0 | 8.8 | 276 | + | 600 | 120 | 74.2 | 8/9 161 | 2.4/0.6 | |||
| Fourth day of hospitalization | 1000 | 300 | 10.6 | 366 | - | 600 | 89 | +3 | 74.71 | 19.85/_ | |||
| Fifth day of hospitalization | 1600 | 656 | 10.9 | 335 | - | 600 | |||||||
| Sixth day of hospitalization | 4700 | 2856 | 11.3 | 417 | - | 600 | 18 | 13 | 76.1 | 2.3/0.3 | |||
| Seventh day of hospitalization | 14030 | 12.4 | 343 | - | 600 | 75.8 | |||||||
| Eighth day of hospitalization | 53600 | 42880 | 12.7 | 438 | - | 300 | 1 | 12 | 75.1 | ||||
| First week after discharge | 56300 | 34906 | 12.1 | 244 | - | - | 74.6 | 2.88 d/1.1 e | |||||
| Second week after discharge | 10300 | 9.6 | 405 | - | 72.5 | 1.05 f | 1.23 g/6.01 h | ||||||
| Second month after discharge | 3800 | 1900 | 11.2 | 221 | - | 71.5 | < 0.005 f | 1.26 g/164 h | |||||
| Fourth month after discharge | 8200 | 5248 | 13.6 | 295 | - | 24.51 f 0.11 i /0.066 e | < 0.3 g/0.56 h | ||||||
| Ten months after discharge | 5800 | 2726 | 12.7 | 239 | - | 4.5 f -/1.76 e |
a mIU/L (0.35 - 5.1).
b Pg/mL (2.5 - 6.5).
c Pmol/L (9 - 23).
d (1.71 - 6.8).
e ng/dL (0.8 - 1.8).
f µIU/mL (0.39 - 4.3).
g ng/mL (0.846 - 2.02).
h µg/dL (5.1 - 12.5).
i Pg/mL (1. - 4.2).
Hyperthyroidism and infection control were the most critical aspects of the patient’s management during admission and after discharge. Although definitive surgical treatment was the best option for this patient, due to severe economic problems, she was treated with 20 millicuries of iodine-131. Almost three months later, she developed iatrogenic hypothyroidism and was treated with a replacement dose of levothyroxine.
3. Discussion
Graves' disease is the most frequent cause of hyperthyroidism in children and is associated with impaired neurodevelopmental outcomes and altered skeletal maturation in younger children, as well as decreased school performance and anxiety in school-aged children and adolescents (7). Methimazole is considered the first-line medication for GD in the pediatric group. Guidelines recommend an initial dose of 0.15 to 0.5 mg/kg/day, with a maximum dose of 30 mg/day for GD treatment, which can be gradually decreased as thyrotoxicosis improves (4).
While most children with GD do not experience therapy-induced complications, methimazole-induced agranulocytosis is a rare but life-threatening adverse effect, with a mortality rate as high as 21.5% (8). Although most patients experience an ANC < 100/μL, drug-induced agranulocytosis is defined as ANC < 500/μL of blood (9). The pathophysiology of methimazole-induced neutropenia is proposed to occur through two mechanisms. The first is direct toxicity, where methimazole is oxidized to reactive metabolites by neutrophils, causing an immune response by activating inflammasomes, which eradicate neutrophils. The second is immune mechanisms, where circulating antibodies against differentiated granulocytes (anti-neutrophil cytoplasmic antibodies) react against specific granules inside the neutrophils, inducing apoptosis and complement-mediated opsonization of neutrophils (6, 10).
Agranulocytosis is reported in 0.2 - 0.5% of patients with GD receiving antithyroid drugs (11). In the pediatric population, neutropenia often occurs within three months after administration in 12.8% of patients, according to a study assessing 304 children with hyperthyroidism hospitalized in China (12).
Identifying the risk factors for agranulocytosis is imperative, but frequent monitoring of the white blood cell count is not cost-effective and is not routinely recommended. Some genetic loci, such as HLA-B*380201 and HLA-B*27:05, are associated with a higher risk of methimazole-induced agranulocytosis (13). A higher female-to-male ratio has been documented in a study from China (14). Risk factors include younger age, lower ANC before treatment, higher doses of methimazole, and female sex (12-15). Our patient was a young female with a positive family history of autoimmune disease.
Symptoms of methimazole-induced agranulocytosis are similar to other causes of neutropenia, such as fever, sore throat, and infections in the oral cavity. In the pediatric population, sepsis should always be considered (6). Our patient had a high-grade fever, sore throat, and diarrhea, which could indicate neutropenic colitis and the risk of colon perforation (16).
Treatment involves the immediate discontinuation of methimazole. Intravenous broad-spectrum antibiotics should be started after collecting samples for blood, urine, and stool cultures. Hospitalization and preventive measures, including good hygiene of the mouth, skin, and perineum, are recommended (10). Granulocyte-stimulating colony factor (G-CSF) has been reported to decrease the duration of neutrophil recovery and hospital stay, though it is not associated with decreased mortality rates. Subcutaneous administration of G-CSF is superior to intravenous injection (17). Determining the appropriate dose of G-CSF based on the age and weight of the patient is crucial, with a recommended dose of 5 mcg/kg/day for febrile neutropenia treatment (18).
After discontinuing methimazole due to agranulocytosis, alternative therapies to control hyperthyroidism must be chosen. Recommended treatments include β-blockers, inorganic iodine, glucocorticoids, bile acid sequestrants, and lithium carbonate (19). β-blockers are the best choice to block sympathomimetic symptoms. Potassium iodide can block the release of T4 and T3 from the thyroid gland via the Wolff-Chaikoff effect and is used as adjunctive therapy in GD with a dose of 2 - 5 drops PO Q6H (20, 21). Glucocorticoids inhibit peripheral T4 to T3 conversion, prevent relative adrenal insufficiency due to hyperthyroidism, and relieve vasomotor symptoms (21). Cholestyramine decreases the enterohepatic circulation of thyroid hormone (TH). Lithium carbonate inhibits TH release by inhibiting the action of TSH on cAMP and can control hyperthyroidism with a recommended dose of 300 - 450 mg PO Q8H (19, 21). SSKI can efficiently control thyrotoxicosis when methimazole and propylthiouracil are contraindicated due to adverse side effects (21).
3.1. Conclusions
Although methimazole is the most routinely used anti-thyroid drug in children, more research is required on its possible adverse effects in the pediatric population. To decrease the risk of agranulocytosis and its potential life-threatening complications, close monitoring of pediatric patients and administering lower doses of methimazole are recommended. Alternative treatments to control hyperthyroidism during methimazole-induced agranulocytosis include β-blockade, solution of potassium iodide (SSKI), cholestyramine, steroids, and lithium.