1. Introduction
1.1. Rationale
Autism spectrum disorder (ASD) is a complex neurodevelopmental syndrome that manifests before the age of 3 years and is characterized by significant impairments in social interaction, communication deficits, and repetitive patterns of interest and activities. The exact cause of ASD remains unknown (1). ASD is thought to result from the interplay of diverse genetic and environmental factors, contributing to its complex behavioral phenotypes (2). Autism is more prevalent in males than females, and the incidence of ASD has been increasing over the years (3). The World Health Organization (WHO) estimates that 1 in 160 children has an autism spectrum disorder (4). With the rising prevalence of ASD in the United States and other countries, many families are seeking treatment options (5). The WHO asserts that mental health is integral to overall health (6). Numerous studies have shown that individuals with autism often suffer from nutritional deficiencies, metabolic imbalances, and gastrointestinal issues.
Diet and nutrition have been identified as beneficial in addressing these conditions (7). In treating ASD, there is growing interest among both parents and healthcare professionals in dietary interventions (8). It has been suggested that peptides from gluten and casein might contribute to the development and symptoms of autism (9). Gluten (from wheat) and casein (from dairy products) are structurally similar proteins (10). A gluten-free diet eliminates all foods containing wheat, barley, and rye (11). Various dietary approaches, such as gluten-free and casein-free diets or the use of probiotics and prebiotics, have been proposed in autism spectrum disorders to alleviate gastrointestinal symptoms (GIs), which are notably prevalent and correlated with the severity of behavioral traits in this population (12). There is increasing interest in gluten-free/casein-free (GF/CF) diets for children with autism spectrum disorder (13). Research indicates a bidirectional communication between the brain and the gut. Children with autism often experience more gastrointestinal disorders, and interactions between the gut and brain microbiome can influence mood and behavior. A gluten- and casein-free diet eliminates specific proteins, such as gluten and casein, from the diet (14). This study was conducted to assess the impact of GF/CF diets on autism spectrum disorder.
In a study involving 45 autistic children, the effects of GF/CF and ketogenic diets were investigated separately. The children were randomly divided into 3 groups: Control, GF/CF, and ketogenic diet. The GF/CF diet group showed significant improvement in language and behavior after 6 months (2). Knivsberg et al. examined the effects of this diet on 20 autistic children in a case-control study over one year; the results indicated that children in the diet group progressed significantly better than those in the control group (15). Conversely, some studies have found no significant impact of this diet on autism spectrum disorder. Hyman et al. explored the safety and effectiveness of the GF/CF diet in 14 autistic children. The results did not show statistically significant effects on physiological measurements, behavioral problems, or autism symptoms (16). Johnson et al. conducted a 3-month trial in 2010 and reported findings based on targeted variables, finding no significant statistical differences between groups in terms of language, behavior, and core symptoms (5).
2. Objectives
In this study, we evaluated all clinical trials conducted among autistic children that focused on the gluten-free/casein-free (GF/CF) diet. We extracted data from accessible articles, specifically including those that provided a detailed assessment of the diet, for our meta-analysis. Our objective was to determine whether, by synthesizing the results of these studies, the GF/CF diet would demonstrate significant improvements in specific behavioral symptoms of autistic children. Given that no previous meta-analysis has been conducted on the impact of the GF/CF diet on the behavior of autistic children, this study was undertaken to explore this topic through a systematic review and meta-analysis."
3. Method
3.1. Protocol and Registration
This article's draft has not been submitted elsewhere, and no meta-analysis on this topic has been conducted previously; therefore, we examined the effectiveness of gluten-free/casein-free (GF/CF) diets on behavioral symptoms in individuals with autism spectrum disorder through meta-analysis. The protocol for this study was registered with Ilam University of Medical Sciences under the number 968040/126. The Autism Treatment Evaluation Checklist (ATEC) is a questionnaire developed by the Autism Research Institute to assess the effectiveness of treatments for autism, consisting of four subscales (2). We adhered to the PRISMA guidelines for conducting and reporting this meta-analysis. In various interventions, exclusion criteria were applied to participants if specific symptoms were observed, and most studies evaluated the safety of the diet before initiating the intervention. Given that many parents already use this diet for their children, it is understood that there is no significant risk associated with the intervention. Parents, doctors, or caregivers closely monitored the children, but we did not encounter any specific dangers related to the 'safety' of the interventions in the studies. Since our intervention involves the effect of a diet and the diet itself is safe, this intervention poses no danger. The signs and symptoms of the patients' behavior were based on a range of factors, including emotion, anxiety, somatic symptoms, withdrawal, sleep, attention, aggression, internalizing, externalizing, effectiveness, anxiety, Pervasive Development Disorder (PDD), Attention-Deficit Hyperactivity Disorder (ADHD), attention, initiation, affective reactions, excitant waiting, intelligible words, and autistic rate.
3.2. Eligibility Criteria
The inclusion criteria for our review were all randomized clinical trials that included the GF/CF diet without a time limit. The exclusion criteria were articles irrelevant to the subject, unavailable articles, and articles that included only a gluten-free or casein-free diet. In different studies included in the meta-analysis, participant inclusion criteria were based on a diagnosis of autistic disorder according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV), Pervasive Development Disorder Not Otherwise Specified (PDD-NOS), Autism Diagnostic Observation Schedule (ADOS), Autism Diagnostic Interview (ADI-R), and Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria.
4. Information Sources
The method was based on a systematic review and meta-analysis using the keywords autism, gluten-free diet, casein, and autistic disorder. We extracted all results from international databases, including Google Scholar, PubMed, Cochrane, and Science Direct, up to August 25, 2023, that included these keywords in the title and abstract. Extracted data included the author's name, publication year, country of origin, sample size in the intervention and control groups, and desired labels data before and after the experiment. Additionally, article references were used to find related articles.
4.1. Search Strategy
We used the following terms to search all databases: Gluten-free, Casein-free, Autism, and autistic. The search strategy in Google Scholar, PubMed, Science Direct, and Scopus included:
(1) Gluten-free
(2) Casein-free
(3) Autism
(4) Autistics
(5) 1 and 2 and 3 or 4
(6) Randomized controlled trials
(7) Randomized-controlled-trial
(8) Controlled-clinical-trial
(9) Random allocation
(10) Double-blind method
(11) Single-blind method
(12) Clinical-trial
(13) 6 or 7 or 8 or 9 or 10 or 11 or 12
5. Study Selection
The evaluation of the inclusion criteria was independently conducted by 2 researchers. Several articles were excluded after a thorough review. Excluding or selecting articles was challenging, as it was necessary to consider the criteria for entering the meta-analysis, and we also had to consult a psychiatrist to determine the relevant labels.
6. Data Collection Process
We developed a checklist for data extraction. One author extracted and entered the data into the checklist, while the second reviewed it. A third author investigated the checklist in case of any mistakes or disagreements. The data extraction was finalized after agreement among the 3 authors.
6.1. Risk of Bias in Individual Studies
Factors such as age at enrollment/outcome measurement, duration of the intervention, and co-interventions could influence the validity of the results. However, in the studies included in the meta-analysis, the participants were children under 16 years old, and most were within a specific age range. Additionally, in the studies incorporated into the meta-analysis, principles of washout were observed in cross-sectional studies, and some studies implemented blinding to reduce bias.
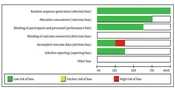
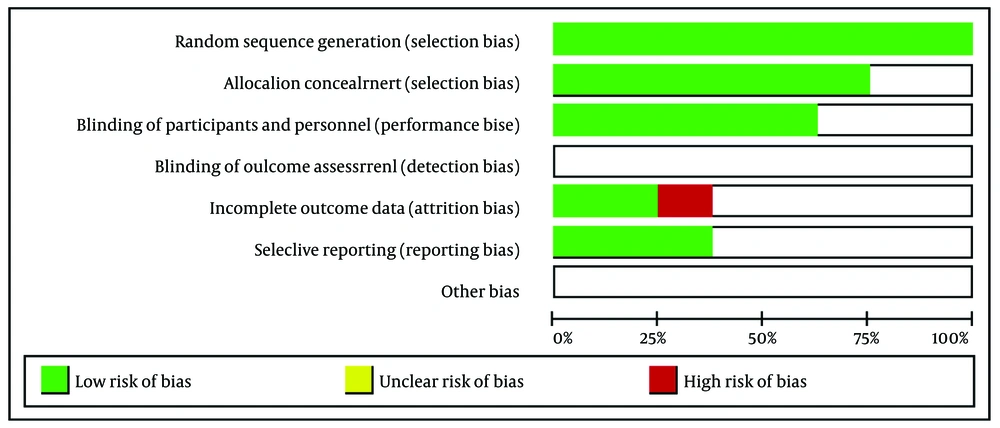
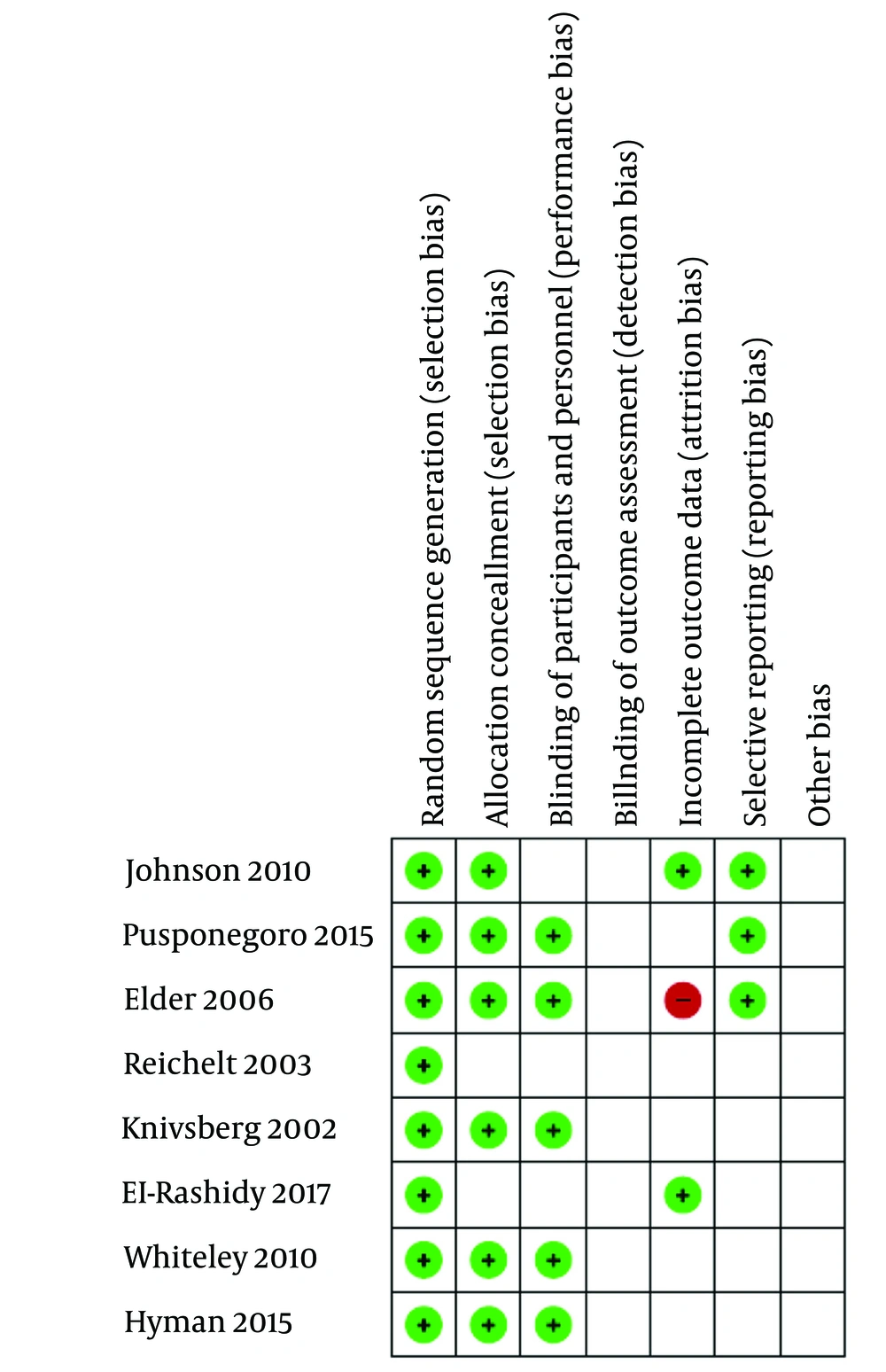
The quality of the included studies in this systematic review was assessed using Cochrane's risk of bias tool within the Review Manager (RevMan) software. We evaluated each study in RevMan based on the following criteria: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. The risks of bias in individual studies are illustrated in Figures 1. and 2.
6.2. Summary Measures
Meta-analysis used the mean and standard deviation before and after the intervention in both case and control groups, employing a fixed-effects model. Effect size and confidence interval were calculated for each label. Meta-analysis was carried out within case groups and between case and control groups (when a control group was present).
6.3. Planned Methods of Analysis
To investigate the relationship between the GF/CF diet and autism spectrum disorder, we utilized the mean and standard deviation of the desired labels from various studies. For each study, the mean and standard deviation before and after the intervention in case and control groups, author, year of publication, sample size, study location, and results were inputted into STATA software for meta-analysis.
The Q statistic and I2 index were used to assess homogeneity between articles. The fixed-effect model was applied if no significant heterogeneity was observed between the studies (I2 < 50%, P > 0.05), and the random-effect model was used in cases of significant heterogeneity (I2 > 50%, P < 0.05). To evaluate the impact of each study, the total effect was calculated by excluding the study from each model.
7. Results
7.1. Study Selection
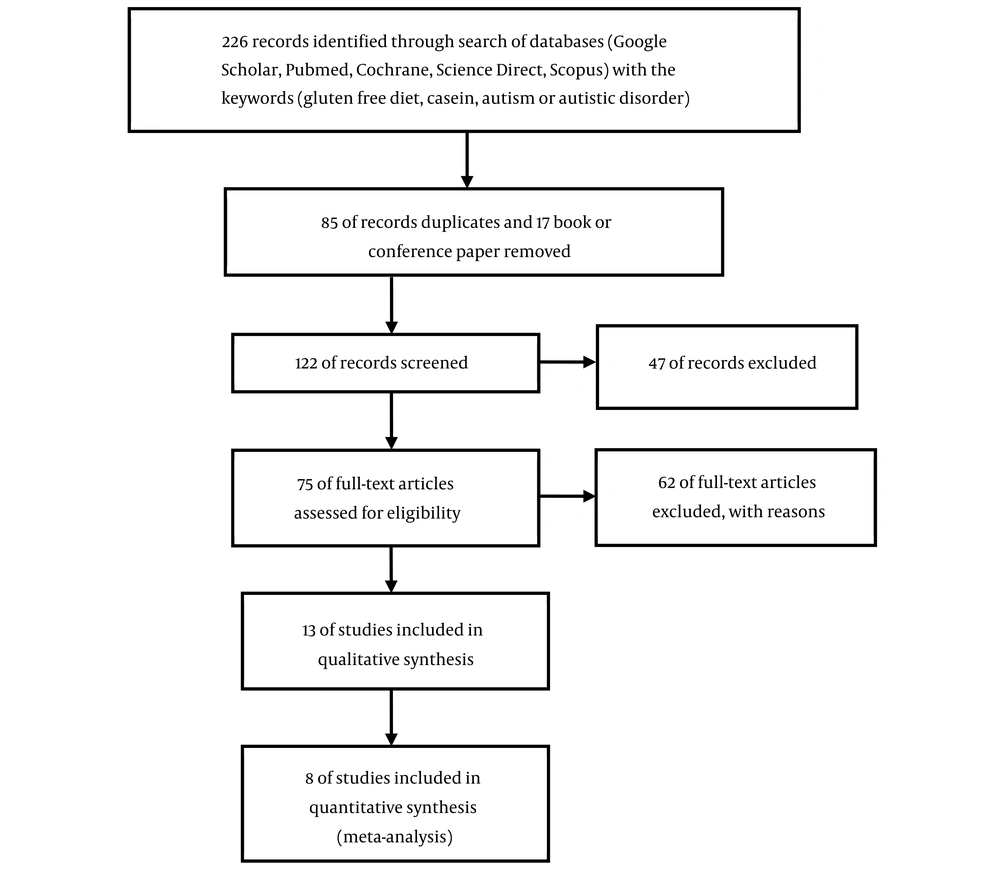
Initially, 226 articles were identified from all international databases using the specified keywords in the title or abstract. After removing duplicates, conference papers, and book chapters, 122 articles remained. A review of the titles and abstracts led to selecting 13 clinical trial articles, with 8 ultimately meeting the eligibility criteria for inclusion in the study. These 8 studies involved 233 autistic children, comprising 128 in the experimental group and 105 in the control group. The details of the search methodology are depicted in Figure 3. Among these 8 studies, 2 were crossover studies, one was a cohort study, and the rest were case-control studies, some of which were double-blind and others single-blind."
The article by Elder et al. included only information about the experimental group (17). The article by Hyman et al., due to its unique design, utilized solely the intervention group information and did not have a control group (16). In the article by Whiteley et al., the standard deviation was not reported; therefore, the mean standard deviation for the target labels from other articles was used in the meta-analysis (13).
7.2. Study Characteristics
The sample sizes in the various studies ranged from 8 to 55 participants. Except for 1 article, the number of participants in the experimental and control groups differed. All participants were children with autism. In 4 of the articles, no significant differences were observed before and after the intervention in the GF/CF diet. In contrast, 4 articles showed a significant difference before and after the intervention in most scales, and there was a significant difference in most scales between the intervention and control groups. Seven articles reported subscales of the behavioral label, 6 articles addressed the subscale of the language label, 5 articles discussed the subscale of the sensory/motor label, and 4 articles focused on the subscale of sociability. The subscales of the behavior label are reported in the syntheses of the results section. More details about the studies included in the systematic review are provided in Table 1. Characteristics of studies qualified for the meta-analysis were entered into STATA ver.14.
| Author | Country/Year | GFCF/Control | Study Design | Age (Rang) GFCF/Control | Intervention Duration |
|---|---|---|---|---|---|
| Hyman et al. (16) | Newyork 2015 | 14 | Prospective, case-control, parallel-group, repeated measures | (3 - 5) years | 30 weeks |
| Johnson et al. (5) | Pittsburgh 2010 | 8/14 | Prospective, case-control, parallel-group | (3 - 5) years | 3 months |
| Knivsberg et al. (15) | Norway 2002 | 10/10 | Prospective, case-control, parallel-group | (62 - 120)/ (59 - 127) months | 1 year |
| Pusponegoro et al. (3) | Indonesia 2015 | 23/24 | Prospective, case-control, parallel-group | (4.8 - 6.7)/ (4.3 - 6.1) years | 7 days |
| El-Rashidy et al. (2) | Egypt 2017 | 15/15 | Prospective, case-control, parallel-group | (3 - 8) years | 6 months |
| Whiteley et al. (13) | Denmark 2010 | 26/29 | Prospective, case-control, parallel-group, repeated measures | (76.6 - 118) / (76.3 - 120.3) months | 12 month |
| Elder et al. (17) | Florida 2006 | 13 | repeated measures, crossover | (2 - 16) years | 12 weeks |
| Reichelt and Knivsberg(18) | Norway 2003 | 14 | Cohort | (2 - 14) years | 1 year |
Details of Studies Included in the Systematic Review
7.3. Syntheses of Results
This meta-analysis showed strong evidence of non-heterogeneity (I2 < 25%). Additionally, no significant difference in findings was noted between fixed versus random effect models. To assess the benefits of the GF/CF diet on the behavior of autistic children, we categorized symptoms according to the Autism Treatment Evaluation Checklist (ATEC) Questionnaire. This categorization allowed us to group all the symptoms reported in various articles into 4 labels. The behavior label encompasses emotion, anxiety, somatic symptoms, withdrawal, sleep, attention, aggression, internalizing, externalizing, effectiveness, anxiety, PDD, ADHD, attention, initiation, affective reactions, excitant waiting, intelligible words, and autistic rate.
The behavior label included 19 symptoms of autistic child behavior. The study by Cynthia reported 15 of these symptoms in its results, necessitating the inclusion of this study 15 times in the meta-analysis of the behavior label. Experts in meta-analysis were consulted to confirm the validity of this approach, and the accuracy of this method was affirmed.
7.4. Behavior
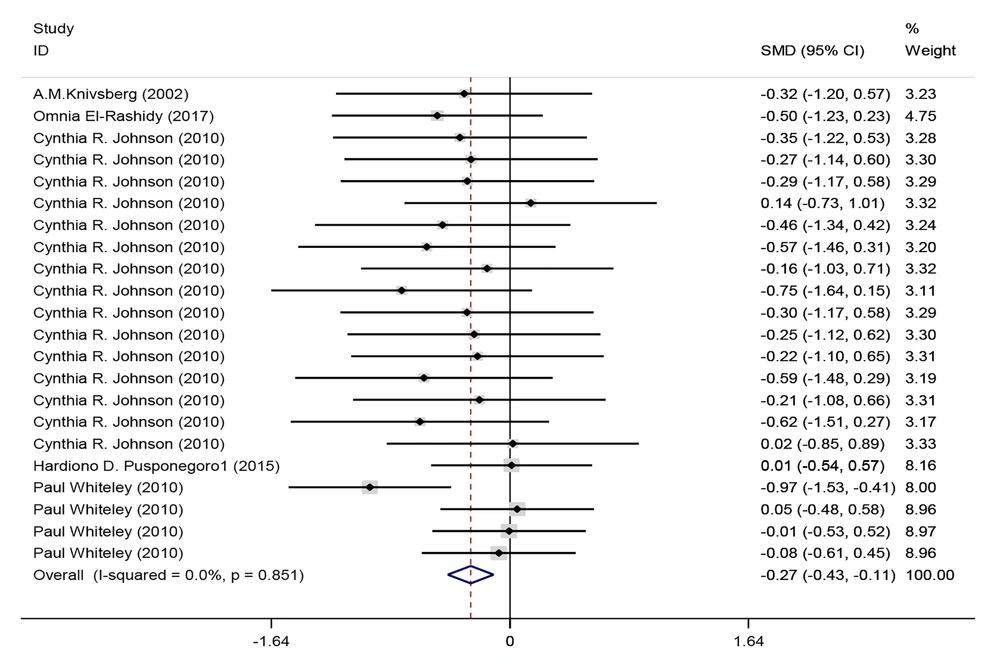
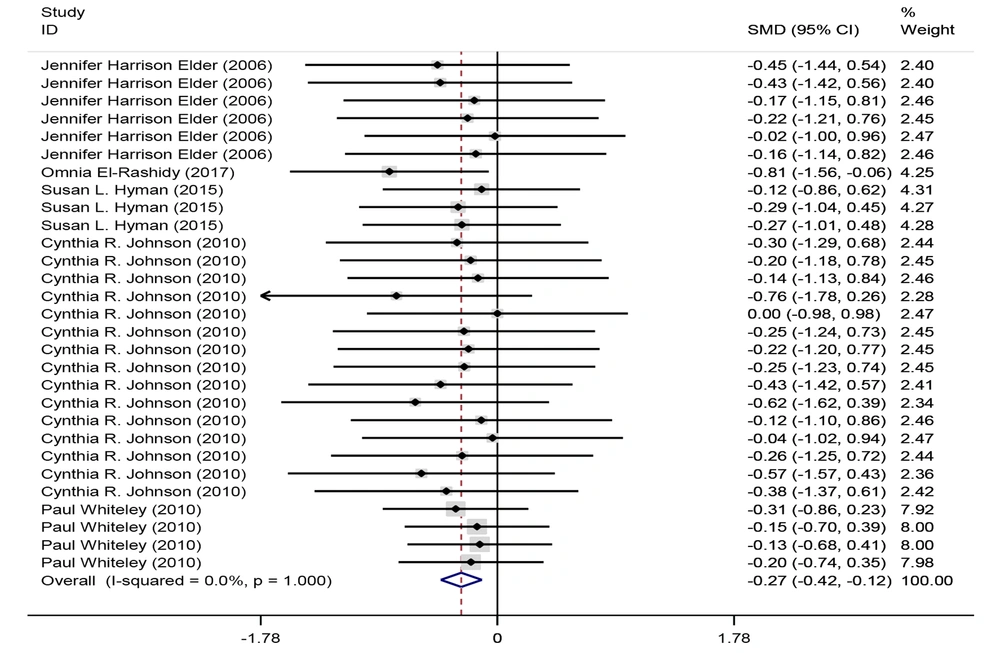
Five studies investigated the subscale of the behavioral label. Most studies demonstrated a positive correlation. Combining the results of these 7 studies between intervention and control groups for meta-analysis, only 5 articles met the criteria for inclusion. Using the fixed effects method (I2 = 0%, P = 0.851), it was determined that the GF/CF diet in children with autism spectrum disorder has an effect size of standard mean difference (SMD) = -0.27 with a confidence interval (95% CI: -0.429 to -0.112) on the behavior label (Figure 4). In a meta-analysis of intervention groups in the behavior label, while excluding three separate subscales, 5 studies were analyzed using the fixed effects method (I2 = 0%, P = 1). In this analysis, the GF/CF diet had an effect size of SMD = -0.27 with a confidence interval of (95% CI: -0.424 to -0.116) (Figure 5 and Table 2).
Forest plots of the comparison of behavior between intervention and control groups following a GFCF diet. Each line in the plot represents the result of an individual study, with the width of the horizontal line indicating the 95% confidence interval (CI), the position of the box representing the standard mean difference (SMD), and the size of the box proportional to the weight of the study. The diamond indicates the pooled effect of the studies.
Forest plots of the comparison of behavior in the intervention group before and after the GFCF diet. Each line in the plot represents the result of an individual study, with the width of the horizontal line indicating the 95% confidence interval (CI), the position of the box representing the standard mean difference (SMD), and the size of the box is proportional to the weight of the study. The diamond indicates the pooled effect of the studies.
| Group and Characteristic | SMD | Lower | Upper | P-Value | No. of Article |
|---|---|---|---|---|---|
| Behavior label in intervention and control groups after GFCF | -0.27 | -0.429 | -0.112 | 0.001 | 22 |
| Behavior label in intervention groups before and after GFCF | -0.27 | -0.424 | -0.116 | 0.001 | 29 |
| Behavior label in control groups before and after GFCF | -0.099 | -0.306 | 0.109 | 0.351 | 22 |
| Behavior label in intervention and control groups before GFCF | -0.052 | -0.21 | 0.106 | 0.52 | 22 |
Effect Size, 95% CI, and I2 Index in Various Labels and Groups
7.5. Risk of Bias Across Studies
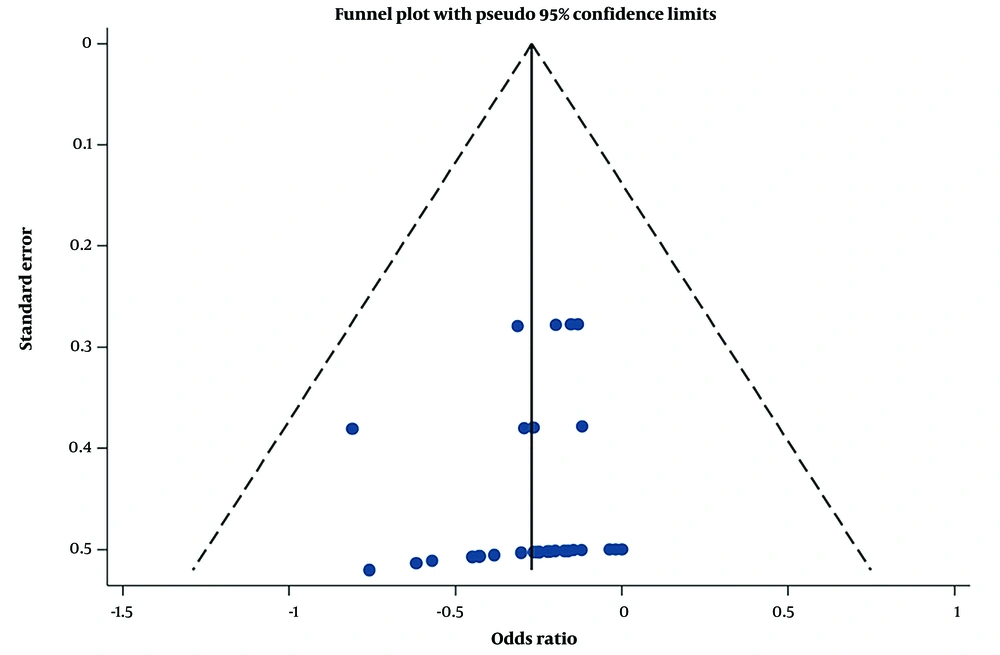
This meta-analysis showed strong evidence of non-heterogeneity (I2 < 25%). To investigate this non-heterogeneity further, funnel plots were created. The funnel plot demonstrates evidence of substantial symmetry, indicating the absence of publication bias. A funnel plot was drawn explicitly for the behavior index (Figure 6).
8. Discussion
8.1. Summary of Evidence
Meta-analysis in this study was conducted to broaden the generalizability of findings and derive convincing conclusions from the results of various similar studies about the effect of a gluten-free, casein-free (GF/CF) diet on maladaptive behavior in autistic children. We performed a meta-analysis to investigate the impact of the GF/CF diet on the behavior of children with autism spectrum disorder. In evaluating this diet in intervention groups across different studies, the diet showed a significant positive effect on the behavior of autistic children. Furthermore, the meta-analysis comparing intervention and control groups indicated that this diet had a significant positive effect size on autistic children's behavior. Therefore, according to these results, reducing the consumption of foods containing gluten and casein is advisable as they may contribute to hyperactivity in autistic children.
One systematic review and meta-analysis study conducted by Hakim, with the following PICO: Population - Autism children, intervention - gluten-free casein-free diet, comparison - no casein-free gluten-free diet, outcome - maladaptive behavior, demonstrated that the casein-free gluten-free diet affects reducing the risk of maladaptive behavior in children with autism. The results of this meta-analysis align with our study's findings (19). Although many studies have examined the effect of the GF/CF diet in children with autism disorders, there is not sufficient evidence for the effectiveness of this diet.
In Reichelt and Knivsberg's study (2003), participants who followed this diet over 1 year showed significant improvements in behavioral ability (18). El-Rashidy et al.'s study reported significant improvements in behavioral, lingual, ATEC, and Childhood Autism Rating Scale (CARS) indicators after 6 months (2). Similarly, Knivsberg et al.'s study (2002), applying this dietary intervention over a year, observed significant positive outcomes in autistic behaviors, non-lexical perceptions, and motor problems (15). However, other studies with shorter treatment durations yielded insignificant results, such as one study over 7 days (3) and another over 3 months (5). Therefore, further evaluations are recommended, considering the short duration of some studies and the need for adherence to complete randomization and blinding principles.
8.2. Limitations
The meta-analysis conducted in this study combined data from multiple studies to estimate the effect of the GF/CF diet on autism spectrum disorder, providing a more impactful result compared to individual studies. As with any overview, the primary limitation of this meta-analysis is the variation in participants, the GF/CF diet, and the definitions of outcomes across the different studies. Our study has several limitations. The quality of the included studies varied. Randomization was adequate in all studies, but four did not mention blinding, 3 were double-blind, and 1 was single-blind. The sample sizes in the studies, except for 2 cases, were small.
Additionally, 2 studies did not have a control group (16, 17). In one study, the standard deviation was not reported (13), and another study had a treatment period of only 7 days (3), which is relatively short. We also did not have access to the full text of some articles (20, 21), excluding several potentially useful studies. Moreover, a more precise result could have been obtained if the exact quantity of the GF/CF diet in the children's diets had been identified.
8.3. Conclusions
Numerous systematic reviews have been conducted on the gluten-free/casein-free (GF/CF) diet, and while all of them have acknowledged the benefits of the diet, the results were not significant. Although this study supports the benefits of this diet in children with autism, it is important to consider the limitations of the studies, such as a low number of participants in some cases, short study durations, and the outdated nature of some interventions. Therefore, a more comprehensive evaluation is recommended in this area. We have previously examined eight clinical trials that were included in the meta-analysis. Given the positive outcomes identified in this study, larger and more randomized controlled trials are necessary.