1. Background
Tic disorder (TD) is a childhood-onset neurodevelopmental condition characterized by sudden, rapid, recurrent, and involuntary contractions of one or more muscles, leading to non-rhythmic, stereotypical movements and/or vocalizations (1, 2). According to the diagnostic and statistical manual of mental disorders, fifth edition (DSM-5), published by the American Psychiatric Association (APA) in 2013, TD can be classified into three groups: Tourette's syndrome (TS), chronic motor or vocal tic disorder (CTD), and provisional tic disorder (PTD) (3, 4). Tic disorder is one of the most common movement disorders in the pediatric population, with a typical prevalence during childhood and adolescence. It affects up to 5% of children worldwide, and approximately 0.52% of children aged 4 to 18 years with TS, with the greatest tic severity typically occurring between 8 and 12 years of age (5-8). Previous studies have shown that TD can lead to both physical and mental health challenges, impacting educational achievement, social relationships, quality of life, and premature loss of self-care ability (5, 9). Despite the identification of multiple risk factors—including genetic, neurological, immune, and environmental factors—the etiology and pathogenesis of TD remain incompletely understood (4, 10). Tics tend to be persistent despite medical and non-medical interventions, with many patients experiencing relapses that continue into adulthood. Risk assessment and management of TD onset and recurrence often rely on biomarkers; however, there is a significant shortage of these resources (11).
Neurodevelopmental disorders, particularly TDs, manifest early in development. From both a clinical and public health perspective, effective and reliable biomarkers are essential for timely diagnosis. Over the past few decades, the limitations of subjective psychiatric diagnoses have spurred numerous studies aimed at identifying valid biomarkers to aid in diagnosis, prediction, prognosis, and management of mental health status (12). If effective, widely available biomarkers are identified, precision medicine could be applied to mental health, offering several advantages: (a) patients would receive accurate diagnoses and appropriate treatments faster; (b) treatment would be better matched to the patient's likelihood of response; (c) early intervention could prevent severe symptoms and functional impairment, increasing the likelihood of recovery; and (d) clinicians could better identify individuals most at risk for recurrence (12).
In recent years, growing evidence has indicated dysregulation of immune responses in patients with TDs (13). A recent study clarified the relationship between circulating cytokines and the occurrence and development of TD through plasma cytokine profiling analysis (3). Tumor necrosis factor-alpha (TNF-α), a key inflammatory cytokine, plays a crucial role in the immune response and, like many other cytokines, is critical for normal central nervous system (CNS) development and function, both in physiological and pathological contexts (14). Notably, TNF-α produced by glial cells can rapidly affect α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor transport, subsequently influencing synaptic strength at excitatory synapses (15). Experimental evidence suggests that presynaptic AMPARs can regulate the release of a wide range of neurotransmitters in addition to GABA and glutamate, including noradrenaline (NA), dopamine (DA), serotonin (5HT), and acetylcholine (ACh) (16). Hyperactivity of striatal DA or hypersensitization of postsynaptic DA receptors has been shown to cause tic symptoms (14). Therefore, we speculate that in the CNS, TNF-α may modulate DA release through AMPA receptor activation, potentially triggering TD symptoms.
Studies have found elevated levels of pro-inflammatory cytokines IL-12 and TNF-α in children with TD and/or obsessive-compulsive disorder (OCD) compared with age-matched controls. These levels were further increased during symptom exacerbation, highlighting the potential role of inflammation in behavioral symptoms (6, 14). However, Singer et al. reported no association between TD symptoms and cytokine levels, particularly TNF-α levels, indicating that more research is needed to fully understand this relationship (13, 17).
Tic disorder has been demonstrated to have strong genetic factors, with common polymorphisms and high heritability (18, 19). Histidine decarboxylase (HDC) is a member of the group II decarboxylase family, which encodes L-histidine decarboxylase and forms a homodimer to convert L-histidine to histamine (HA) in a pyridoxal phosphate-dependent manner (20). In a groundbreaking study, Ercan-Sencicek et al. conducted a genome-wide analysis on DNA samples from two generations of a family with a high incidence of Tourette syndrome (TS). They identified a rare terminating mutation, W317X, in exon 9 of the HDC gene, which completely abolishes the function of HDC in biosynthesizing HA from histidine and has been implicated as a rare genetic cause of TS (21, 22). Studies have shown that HDC knockout (HDC-KO) and heterozygous mice exhibit core features of TS, including increased stereotypy and dysregulation of D2 and D3 receptors. Notably, pretreatment with haloperidol or HA injections relieved stereotypy in HDC-KO mice (22). These findings suggest that HDC may play a role in the pathogenesis of TD by regulating HA synthesis.
2. Objectives
However, the correlation between serum HDC levels and TD has not yet been explored, and there are limited data on serum TNF-α levels in TD patients, with some of the results being inconsistent. To address this gap, our retrospective study aimed to investigate the role of elevated serum HDC and TNF-α as potential biomarkers in children with TDs and their association with high risk. The goal is to enhance our understanding of the pathogenesis of TD and provide a new perspective for early diagnosis, patient evaluation, and the development of targeted interventions, thereby laying the foundation for optimal treatment of TDs.
3. Methods
3.1. Study Design and Participants
A total of 118 patients (median age 6.76, range 2.83 - 12 years) with TDs who were treated at our hospital and 111 healthy, age-matched children (median age 6.60, range 2.17 - 11.5 years) who underwent outpatient physical examinations from April 2021 to May 2023 were enrolled. The TD patients were diagnosed based on the clinical characteristics and course of the disease described in the DSM-V criteria and were categorized into three types: TS (n = 29), CTD (n = 31), and PTD (n = 58) (23). Subsequently, the TD patients were divided into two groups: A mild group (n = 68, score < 25) and a moderate-to-severe group (n = 50, with a score of 25 - 50 considered moderate and a score > 50 considered severe) based on tic severity, which was evaluated using the Yale Global Tic Severity Scale (YGTSS) (24). The study was approved by the Ethics Committee of the Hospital with the ethical approval code 2023-K-131 and strictly adhered to the relevant provisions of the Helsinki Declaration. Informed consent was obtained from the parents or legal guardians of all participants.
3.2. Inclusion and Exclusion Criteria
- Inclusion criteria: Participants were considered eligible if they met the DSM-V diagnostic criteria for childhood TDs (25, 26), could participate in daily life and school activities, had not taken medication for 1 year prior to enrollment, and the family members of the participants were informed and agreed to the trial process.
- Exclusion criteria: Participants were deemed ineligible if they had any known comorbidities, including other hereditary, serious organic, nervous system, or mental diseases.
3.3. Detection of Biomarkers in the Plasma
Serum samples were collected at study entry for the healthy children, while for the TD group, serum samples were obtained at the time of tic onset. The collected blood was immediately processed into plasma and stored at -80°C for subsequent analysis. Serum HDC and TNF-α levels were collected and analyzed by two experienced observers who were blinded to the patients’ clinical status. Plasma HDC and TNF-α levels were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits from mlbio, according to the manufacturer’s instructions [HDC (ml060416), TNF-α (ml077385)].
3.4. Statistical Analysis
All data were analyzed using SPSS 23.0 statistical software. Categorical data were presented as percentages and compared using the chi-square test. Continuous data were presented as mean ± standard deviation. Depending on the distribution of the analyzed variable, significance testing for differences between mean values of two or more continuous variables was performed using Student's t-test, the non-parametric Mann-Whitney test, or one-way ANOVA. Correlations between tic severity and serum HDC and TNF-α levels were evaluated using Pearson’s correlation test. Binary logistic regression analysis was conducted to assess the association of TD occurrence with gender and serum HDC and TNF-α levels, and results were expressed as odds ratios (OR) with 95% confidence intervals. The predictive values of serum HDC and TNF-α levels were evaluated using receiver operating characteristic (ROC) curve analysis, and the area under the curve (AUC) was calculated accordingly. Two-tailed tests were used for all comparisons, with statistical significance assumed at P < 0.05.
4. Results
4.1. Patient Characteristics and Concentrations of Each Biomarker in Tic Disorder Patients and Healthy Controls
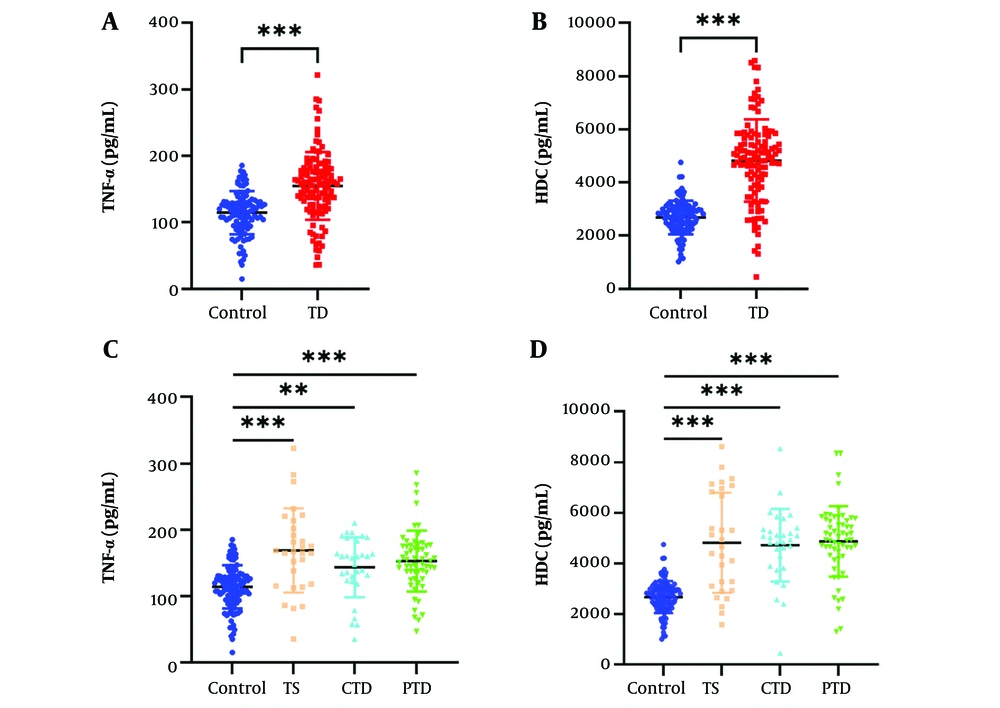
To ensure the accuracy of our analysis was not affected by comorbidities or prior medication, comorbid patients and those recently medicated were excluded from the study during the enrollment of TD patients. There was no significant difference in age between the TD patients and healthy controls (P > 0.05); however, the proportion of boys in the TD group was significantly higher than in the control group, consistent with previous reports (3, 27). Additionally, the BMI of the TD group was significantly higher than that of the control group. The total tic severity scores were 23.70 ± 6.63, with motor tic scores of 9.44 ± 3.18, vocal tic scores of 6.24 ± 3.64, and impairment scores of 8.03 ± 4.38 (Table 1). To systematically compare the relative expression levels, we employed ELISA to measure TNF-α and HDC levels in the plasma of TD patients and controls. The results showed that the mean serum level of TNF-α in TD patients was significantly higher than in the controls (Figure 1A and C). Similarly, the mean serum level of HDC was elevated to varying degrees in TD patients compared to controls (Figure 1B and D).
| Variables | Control (n = 111) | TD (n = 118) | t/χ2 | P-Value |
|---|---|---|---|---|
| Age (y) | 6.60 ± 2.22 (2.17 - 11.5) | 6.76 ± 2.06 (2.83 - 12) | 0.547 | 0.85 |
| Sex; male | 57 (51.4) | 93 (78.8) | 224.047 | < 0.001 |
| YGTSS | - | 23.70 ± 6.63 | - | - |
| Motor tics | - | 9.44 ± 3.18 | - | - |
| Vocal tics | - | 6.24 ± 3.64 | - | - |
| Impairment | - | 8.03 ± 4.38 | - | - |
| BMI | 19.87 ± 5.62 | 25.13 ± 6.23 | 6.716 | < 0.001 |
Abbreviations: TD, tic disorder; YGTSS, Yale Global Tic Severity Scale.
a Values are shown as No. (%) or mean ± standard deviation (SD).
The level of plasma tumor necrosis factor-alpha (TNF-α) and histidine decarboxylase (HDC) increased in tic disorder (TD) patients. The level of serum TNF-α (A) in control group and TD group measured by the enzyme-linked immunosorbent assay (ELISA) assay (t = 7.183, P < 0.001). The level of serum HDC (B) in control group and TD group measured by the ELISA assay (t =13.868, P < 0.001). The level of serum TNF-α (C) in healthy controls and TD patients (TS, CTD, PTD) measured by the ELISA assay (t = 4.494, P < 0.001, t = 3.393, P = 0.002, t = 5.750, P < 0.001).The level of serum HDC (D) in healthy controls and TD patients (TS, CTD, PTD) measured by the ELISA assay (t = 5.773, 7.756, 11.453, P < 0.001).** P < 0.01, *** P < 0.001.
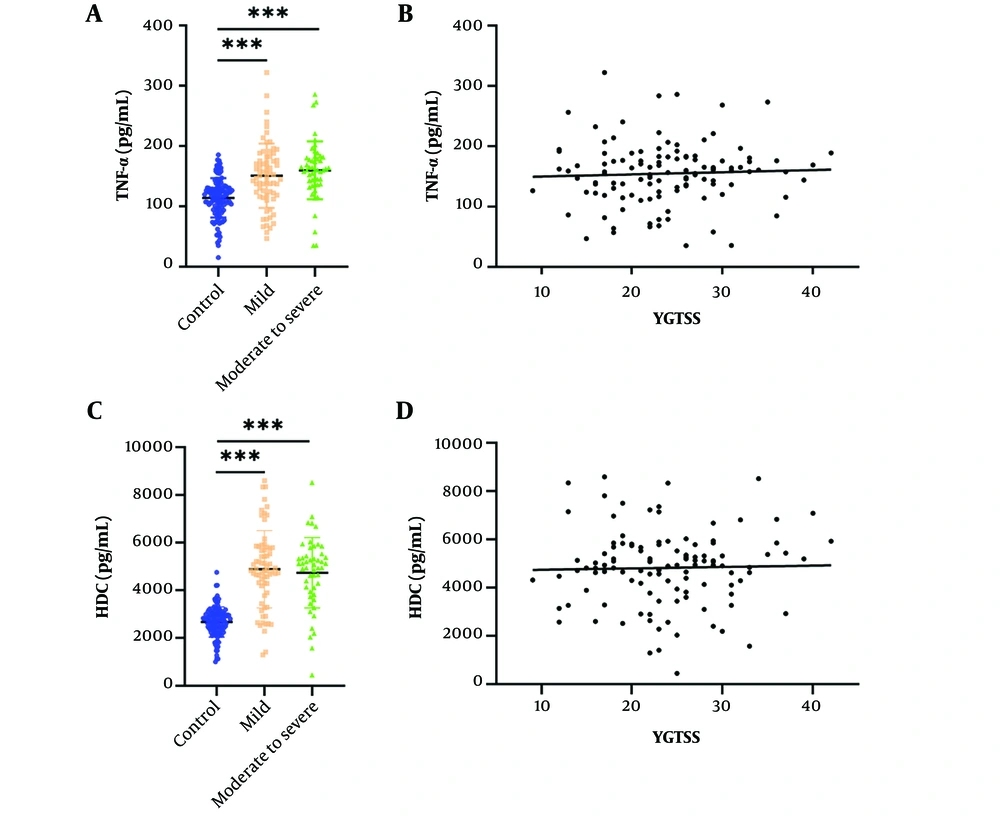
Comparison of the Control, Mild, and Moderate-to-Severe Groups in Terms of Biomarkers. All patients were divided into groups according to the methods described above, with 68 cases classified as having mild tic symptoms and 50 cases as having moderate-to-severe symptoms. The mean levels of TNF-α and HDC were significantly higher in both the mild and moderate-to-severe groups compared to the control group (Figure 2A and B). The association between TNF-α, HDC, and tic severity, as assessed by the YGTSS in TD patients, was measured using Pearson's correlation test, which revealed no significant correlation (Figure 2C and D).
The level of plasma tumor necrosis factor-alpha (TNF-α) and histidine decarboxylase (HDC) increased in mild and moderate to severe tic disorder (TD) patients and correlations with tic severity of all TD patients. The level of serum TNF-α (A) in control, mild and moderate to severe TD groups measured by the enzyme-linked immunosorbent assay (ELISA) assay (t = 5.112, 6.114, P < 0.001). The level of serum HDC (B) in control, mild and moderate to severe TD groups measured by the ELISA assay (t = 10.787, 9.525, P < 0.001). The correlations of plasma TNF-α (C) and HDC (D) with tic severity of all TD patients assessed with the YGTSS. *** P < 0.001.
4.2. Diagnostic Value of Each Biomarker in Tic Disorders
To assess the diagnostic performance of TNF-α, HDC, and gender—which also showed a significant difference between TD patients and healthy controls (Table 1)—binary logistic regression analysis was conducted. The analysis revealed that gender, TNF-α, and HDC significantly contributed to the development of TD. According to Table 2, the observed incidence of TD in boys was 3.671 times higher than in girls, which is consistent with the previously reported gender preference in TD (3, 28). Regarding HDC and TNF-α, the analysis indicated that for every 10 pg/mL increase in HDC and TNF-α concentration, there was a 2% and 24% increased risk of TD development, respectively (Table 2), suggesting that HDC and TNF-α may be potential risk factors for TD.
| Variables | OR (95% CI) | P-Value |
|---|---|---|
| Gender | < 0.001 | |
| Female | 1.00 | |
| Male | 3.671 (2.049 - 6.576) | |
| HDC (pg/mL) | 1.002 (1.001 - 1.003) | < 0.001 |
| TNF-α (pg/mL) | 1.024 (1.016 - 1.032) | < 0.001 |
Abbreviations: HDC, histidine decarboxylase; TNF-α, tumor necrosis factor-alpha.
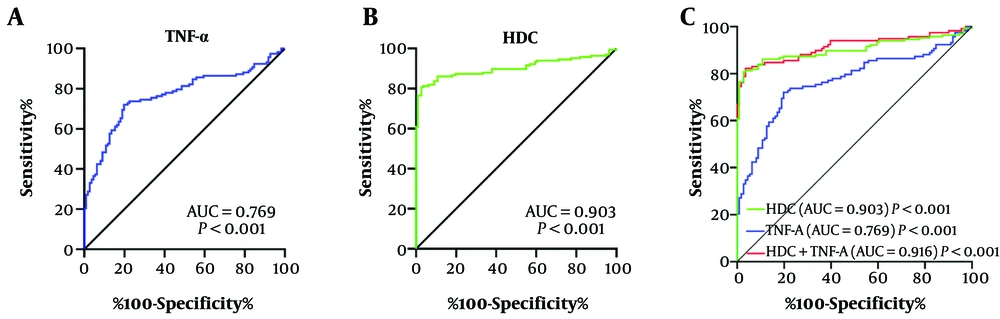
The diagnostic value of serum TNF-α, HDC, and combined markers for TDs was assessed using ROC curve analysis. The area under the curve (AUC) for TNF-α was 0.769, with 72% sensitivity and 80% specificity (Figure 3A). The AUC for HDC was 0.903, with 80% sensitivity and 97% specificity (Figure 3B). The AUC for the combined marker was 0.916, with 82% sensitivity and 96% specificity (Figure 3C). The diagnostic performance of the combined marker was better than TNF-α and HDC alone. These results suggest that HDC and TNF-α could be promising predictors for the risk of developing TD.
The detected performance of tumor necrosis factor-alpha (TNF-α), histidine decarboxylase (HDC) and combined marker for tic disorders. Receiver Operating Characteristic (ROC) curves of TNF-α, A; HDC, B; and the combined marker, C; for discriminating between tic disorder (TD) group and Control group.
5. Discussion
In previous studies, various abnormal serum proteins have been identified in patients with TD (14, 29, 30). Parker-Athill et al. found that seventy-seven percent of patients expressed significantly higher levels of serum TNF-α during periods of tic symptom exacerbation (14). Bombaci et al. provided the first evidence that tic patient sera exhibit immunological profiles typical of individuals with a broad, specific, and strong immune response against Group A Streptococcus antigens (29). Yeon et al. showed that interleukin-12 p70 (IL-12p70) and TNF-α levels increased in the patient group compared to healthy controls, with the patient group having an anti-streptolysin O (ASO) score under 200 or YTGSS score from 10 to 19 also showing higher IL-12p70 or TNF-α levels (30).
However, to our knowledge, while these serum proteins have been confirmed to be related to TD, they have not yet been identified as risk factors with a clear association, and their diagnostic effectiveness is unknown. In this study, commercial ELISA kits were used to measure HDC and TNF-α levels in TD patients without comorbidities or medication, as well as age-matched healthy controls. Additionally, the correlation between these proteins and the development of TD was evaluated. For the first time, we found that two key elevated serum proteins, HDC and TNF-α, were significantly increased in the serum of TD patients and confirmed their association with TD. More importantly, we discovered that the average levels of HDC and TNF-α showed a significant increasing trend in TD patients with mild and moderate-to-severe symptoms compared to age-matched healthy controls. Furthermore, ROC curve analysis demonstrated that HDC and TNF-α could serve as potential risk factors for TD, despite showing no correlation with YGTSS scores. Thus, peripheral blood HDC and TNF-α may be important indicators for assessing susceptibility to TD development.
The etiology of TD appears to be complex and multifactorial, with increasing evidence supporting its association with various immunological diseases, such as autoimmune responses induced by streptococcal infection and other autoimmune conditions. Notably, theories suggesting that infection can regulate the manifestation of tic symptoms date back to the 19th century (3, 13, 31). Infection and immune dysfunction have long been considered central components of TDs, partly due to the strong similarities with Sydenham’s chorea (SC), which is triggered by infection, and the observation of infection-induced symptoms, cytokines, and other immune abnormalities (14). Other studies have revealed overexpression of natural killer and cytotoxic T-cell genes in TD and comorbid ADHD through cellular microarray gene expression profiling (32, 33). Additionally, a recent study indicated that synaptic processes and immune-related pathways are implicated in TS, based on genome-wide genotypic data analysis (34). These findings highlight the link between dysregulated immune responses and TD development and suggest that numerous cytokines may function as key effectors and regulators involved in the pathogenesis of TD.
As a powerful regulator of the immune system and inflammatory processes, TNF-α recruits macrophages to sites of inflammation, activates T-cells, and promotes their differentiation, as well as induces downstream cytokines and other immune mediators that play key roles in inflammation and immune response (35, 36). Like many other cytokines, TNF-α also plays a crucial role in the physiological and pathological regulation of the CNS (14). Recent studies on amyotrophic lateral sclerosis (ALS) have focused on the pathological features of the condition, showing that TNF-α levels in patient serum were already elevated before the onset of clinical manifestations of motor dysfunction (37). Juedes et al. demonstrated that IL-12 and TNF-α play key roles in local brain inflammation in an animal model of multiple sclerosis induced by myelin oligodendrocyte glycoprotein (MOG) (38). Groh et al. also observed increased TNF-α levels in a cohort of depressive patients (39).
However, the expression of TNF-α in the peripheral blood of children with TD remains a topic of debate. In contrast to the findings reported here, one previous study found no significant difference in plasma TNF-α levels between pediatric TD patients and controls (40). Nevertheless, our results are consistent with an earlier study reporting that serum levels of IL-12 and TNF-α were higher in children with TD and/or OCD compared to age-matched controls, with further increases during symptom exacerbations (31). These discrepancies may be attributed to methodological differences in study design, including variations in the patient populations selected by each institution. Although patients in most studies were included based on a TD diagnosis, the presence of comorbid disorders and differences in criteria defining flare-ups versus remission may contribute to the divergent findings.
In peripheral tissues, TNF-α is constitutively expressed by macrophages, circulating myeloid cells, and NK cells, whereas in the brain, it is primarily derived from neurons, astrocytes, and microglia (41). There is substantial evidence supporting the interaction between peripheral and central cytokine expression, such that TNF-α messenger RNA expression is induced by peripherally injected lipopolysaccharide (LPS) in perivascular cells, meningeal cells, and neurons in various brain regions (41, 42). Additionally, TNF-α plays a role in recruiting leukocytes through the blood-brain barrier (BBB), which may allow B-cells and other immune cells to enter the CNS (43). Notably, TNF-α produced by glial cells can rapidly affect α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor transport, thereby influencing synaptic strength at excitatory synapses (15). Thus, once TNF-α levels are elevated in the CNS, it can significantly alter neuronal activity, including the DA release process following local AMPA receptor activation, potentially triggering TD symptoms.
Furthermore, several studies have shown that TNF-α can induce excitotoxicity by promoting glutamate signaling and the expression of GABA and glutamate receptors (44, 45). In ALS, highly expressed TNF-α is associated with symptom severity and involves the GABA system. Given the clinical similarities between TD and ALS, the role of TNF-α in ALS may provide insights into its role in TDs (46, 47). This suggests that in TD, elevated TNF-α levels may produce excitotoxicity by regulating the GABA system, which could subsequently trigger the TD phenotype.
Neuronal HA is produced from L-histidine by HDC, the key enzyme for HA production, in the tuberomammillary nucleus (TMN) of the posterior hypothalamus. Studies have shown that HDC plays an important role in neurological diseases. Shan et al. found that HDC-mRNA levels in healthy controls were significantly higher during the daytime than at night, while this pattern was markedly different in patients with neurodegenerative diseases such as Parkinson's disease (PD), Alzheimer's disease (AD), and Huntington's disease (HD), all of which are characterized by sleep-wake disturbances. The diurnal fluctuation of HDC-mRNA expression in the human TMN supports a role for neuronal HA in regulating day-night rhythms (48). Additionally, Acevedo et al. demonstrated that HDC-deficient female mice exhibited hypoactivity, increased anxiety, and impairments in water-maze performance. These findings support an important role for HA in anxiety and cognition and highlight the importance of considering sex differences when interpreting the effects of deficient histaminergic neurotransmission on brain function (49).
In recent years, the HDC gene has become a topic of intense study in the field of TS research. Ercan-Sencicek et al. first reported that the W317X mutation in HDC is associated with the susceptibility of a two-generation pedigree to TS, highlighting the importance of this gene in the molecular mechanism of TS (21). Subsequently, Fernandez et al. conducted a case-control study involving 460 TS patients and 1,131 healthy controls and found gene enrichment in histaminergic signaling pathways by comparing genomic copy number variants (CNVs) (50). Moreover, single nucleotide polymorphism (SNP) analysis revealed that rs1894236 may be directly involved in the transcriptional regulation of HDC in a large TS sample of 520 families from seven European countries (51). Additionally, HDC-KO and heterozygous mice exhibited core features of TS, including increased stereotypy and dysregulated D2 and D3 receptors, while haloperidol pretreatment or HA injections alleviated stereotypy in HDC-KO mice (22). These findings demonstrate the susceptibility of TS to HDC mutations, providing further evidence to support the close relationship between HA dysregulation and TS.
To date, the role of the HDC/HA axis in TDs remains unclear. Previous studies have shown that HA has a negative regulatory effect on DA (52, 53). Recently, Baldan et al. demonstrated a compensatory dysregulation of DA D2+D3 receptors within the basal ganglia of TS patients carrying the HDC W317X mutation, reflecting an imbalance in dopaminergic regulation. They also found that HA levels in HDC-KO mice were significantly reduced, while DA levels were significantly elevated, further supporting DA dysregulation in patients with mutated HDC (22).
From a pathological standpoint, TD is believed to result from abnormal interactions within and between different brain regions, particularly the basal ganglia-cerebellar-thalamo-cortical (BGCTC) network. The BGCTC network, which plays a crucial role in inhibiting undesired behavior, is key to the pathogenesis of tics (54). Related studies suggest that excessive DA release in the striatum is a primary cause of BGCTC dysfunction in TD, and DA D2 antagonists, which target DA-induced effects, are currently the most effective drugs for treating TDs (10, 55). Therefore, it is reasonable to infer that the HDC/HA axis may regulate the development of tics by modulating the striatal DA/BGCTC pathway.
Although the cause of increased HDC levels in the serum is not clear, at least two possibilities exist. One possibility is that the HDC in the serum originates from the brain. In patients with TDs, brain stress may lead to the production of high levels of HDC, and the synthesized HA may directly interfere with the DA/BGCTC axis to control the occurrence of tics. Additionally, HDC produced in the brain could leak into the peripheral blood, resulting in elevated serum HDC levels. Another possibility is that the elevated serum HDC originates from other tissues. How the increased serum HDC contributes to disordered brain function remains to be further investigated.
Due to the structure of the BBB, cerebral microvascular endothelial cells control the entry of cells and harmful agents into brain tissue (56), making it unlikely that serum HDC directly exerts significant adverse effects on the CNS under normal conditions. Interestingly, studies have shown that HDC and its product, HA, are involved in modulating BBB permeability and the entry of immune cells (57-59). For example, Beghdadi et al. demonstrated that the resistance of HDC-KO mice to cerebral malaria (CM) was associated with preserved BBB integrity, the absence of infected erythrocyte aggregation in cerebral vessels, and a lack of sequestration of CD4 and CD8 T-cells (57). Additionally, while the BBB is generally not easily infiltrated by HA, HA can induce an increase in BBB permeability, thereby facilitating its entry into the CNS (58, 59). These findings suggest another possibility: That circulating HDC and HA may exert regulatory effects within the brain. In exploring the pathogenesis of TDs, it is particularly interesting to investigate whether elevated circulating HDC and HA decrease BBB permeability or increase HDC and HA levels in the brain. This may further enhance our understanding of the pathogenesis of TDs and provide additional clues for identifying potential therapeutic targets.
According to the available evidence, the neurotransmitter HA in the brain plays a role in regulating brain function by controlling glial cells and neurons, thereby influencing the CNS (60, 61). Recent studies show that HA-related agents have a positive effect in alleviating abnormal symptoms in animal models of human diseases, including ALS (62), PD (63), major depressive disorder (MDD) (64), and brain ischemia (65). Pharmacological studies suggest that enhancing central HA production can regulate stereotypy, the core phenomenology of TD, induced by methamphetamine, apomorphine, or HDC deficiency (22). These studies suggest that alterations in the HDC-HA-HA receptor cascade in the brain may offer a promising therapeutic option for CNS-related neurological disorders.
Microglia are resident immune cells of the CNS and play a crucial role in neuronal survival and the maintenance of neurogenesis (66, 67). Several studies have found localized inflammatory microglial activation in the striatum of patients with TS (68, 69). Frick et al. constructed the HDC-KO mouse, which reproduces the human TS symptomatology, and found that while inflammatory markers were normally expressed, the arborization of microglia and the number of microglia expressing insulin-like growth factor 1 (IGF1) were reduced. Insulin-like growth factor 1 is essential for the survival and formation of neurons, suggesting that the neuroprotective effect of microglia may be impaired in this model (66, 70). Furthermore, bacterial LPS significantly induced microglial activation and enhanced the expression of pro-inflammatory cytokines TNF-α and IL-1β in the striatum of HDC-KO mice compared with wild-type controls. These animal experiments suggest that microglia-mediated neuroprotective defects, as well as an overresponse to environmental challenges, may exist in TD. This may provide valuable insight into the HDC and microglia-related pathomechanisms in TD.
In light of our findings, we provide the first insight suggesting that HDC and TNF-α could be potential biomarkers and therapeutic targets for TDs. With the rapid development of gene therapy, the world's first SMA-targeted drug, Nusinersen sodium injection, has been successfully used for spinal muscular atrophy (SMA) treatment. This drug is delivered directly to the cerebrospinal fluid (CSF) via intrathecal injection, improving motor function, survival, and altering the disease course of SMA. This suggests that precisely targeted therapeutic agents aimed at addressing HDC deficiency may also improve motor function and alter the disease course of TDs. Given the recurring nature of TDs, monitoring serum HDC and TNF-α levels, while pharmacologically increasing HA levels (biosynthesized by HDC) and reducing serum TNF-α levels, could more effectively control the risk of TD recurrence.
Increasing evidence supports that serum protein measurements may serve as an important clinical tool for determining susceptibility, diagnosis, symptom monitoring, and guiding therapeutic interventions. However, more research is needed to elucidate the exact role of serum proteins in the etiology of TD. Although this study supports the hypothesis that serum HDC and TNF-α are risk factors for TD development, there are several limitations, including the relatively small sample size and the use of a single cohort of patients. Future research should involve systematic analysis and functional validation in multicenter studies with larger cohorts to confirm these findings.
5.1. Clinical Significance
Despite its limitations, this study supports previous findings by other research groups reporting serum protein dysregulation in patients with TD. Furthermore, it identifies TNF-α and HDC as important targets for investigating cytokine dysregulation, abnormal immune function, and dysregulated histaminergic pathways in the TD population. Understanding the role of immune and histaminergic pathway abnormalities in the pathogenesis of TD will not only enhance our understanding of the mechanisms underlying this disorder but may also provide valuable biological markers for diagnosis, assessment of symptom severity, and therapeutic intervention.