1. Background
Type-1 Diabetes Mellitus (T1DM) results from autoimmune destruction of pancreatic β-cells, leading to alterations in bone metabolism, including slowed bone development and osteoporosis (1). The pathomechanisms affecting bone development in T1DM patients involve factors such as decreased bone turnover, low levels of insulin-like growth factor-1 (IGF-1), an impaired PTH-vitamin D axis, inflammatory cytokines, and microvascular diseases induced by the disease (2). Additionally, bone mineral density (BMD) may decrease due to insulin deficiency and reduced production of IGF-1 (3). Skeletal development in children is heavily influenced by environmental and hormonal factors, and since most cases of T1DM are diagnosed during adolescence, a critical period for skeletal development, this can have significant implications (4). Although previous research has explored the effects of T1DM on bone structure, there is a paucity of studies specifically examining pediatric patients with T1DM.
Panoramic images have been utilized to predict patients with low BMD (5). The correlation between the mandible and BMD has garnered increased attention in recent years, with radiomorphometric indices showing a strong association (5, 6).
Fractal analysis (FA) is an image analysis method used to characterize complex shapes and structures, increasingly applied in scientific research for analyzing biological images in various fields. In dentistry, FA proves useful in describing the intricate structure of trabecular bone (7).
2. Objectives
This study aims to investigate changes in the bones of adolescents with T1DM using fractal dimension (FD) analysis method and panoramic radiomorphometric indices.
3. Methods
3.1. Study Group
All examinations and practices were approved by the local ethics committee and were conducted in accordance with the Helsinki Declaration of 1964 and its subsequent versions (No: 441/2022). According to Limeira et al. (8), G*Power analysis determined that at least 35 children should be included in each group, with a 95% confidence interval and 80% power.
The patient group consisted of 36 children diagnosed with T1DM. Oral examinations and radiological imaging were performed on the day of T1DM diagnosis. Diagnosis of T1DM was confirmed by assessing fasting blood glucose, postprandial blood glucose, HbA1c levels, and the presence of autoantibodies such as insulin antibodies in the blood. The control group comprised randomly selected individuals matched for age range and gender with the T1DM group. Body mass indexes (BMI) were recorded. None of the patients had any systemic diseases affecting bone metabolism, such as malnutrition, malabsorption, hormone disturbances, vitamin D insufficiency, or were using medications affecting bone metabolism.
3.2. Panoramic Images
Panoramic radiographs of each participant were taken using a panoramic dental X-ray machine (ProMax®; Planmeca, Oy, Helsinki, Finland) with standardized imaging parameters (62 kVp, 4 mA, 16.2 s) and positions recommended by the manufacturer.
3.3. Fractal Analysis
The box-counting technique developed by White and Rudolph (9) was used for FD. Right and left regions of interest (ROI) were selected in the ramus, angulus, and corpus regions of the mandible (Figure 1).
A, selection of regions of interest (ROI) on panoramic radiography for use in fractal analysis (FA) [385 × 194 mm (120 × 120 DPI)]; B, fractal dimension (FD) analysis transactions; a, duplicated ROI; b, blurred image duplicated ROI; c, the blurred image was then subtracted from the original image; d, adding 128 to the result; e, application of 128 threshold value; f, erosion process; g, dilatation process; h, reversing; I, skeletonizing [237 × 119 mm (120 × 120 DPI)]; C, dividing image sizes into squares in specified pixels; D, inclination of the line showing the FB Value and analysis data for FB.
Fractal analysis procedures were conducted by a single investigator using ImageJ 1.52b software (National Institutes of Health, Bethesda, MD) for image analysis.
3.4. Radiomorphometric Measurements
Radiomorphometric measurements were presented in a table and conducted using a medical imaging program (Turcasoft Medical Viewer, Trabzon, Turkey) on panoramic radiographic images (Table 1 and Figure 2).
| Index | Description |
|---|---|
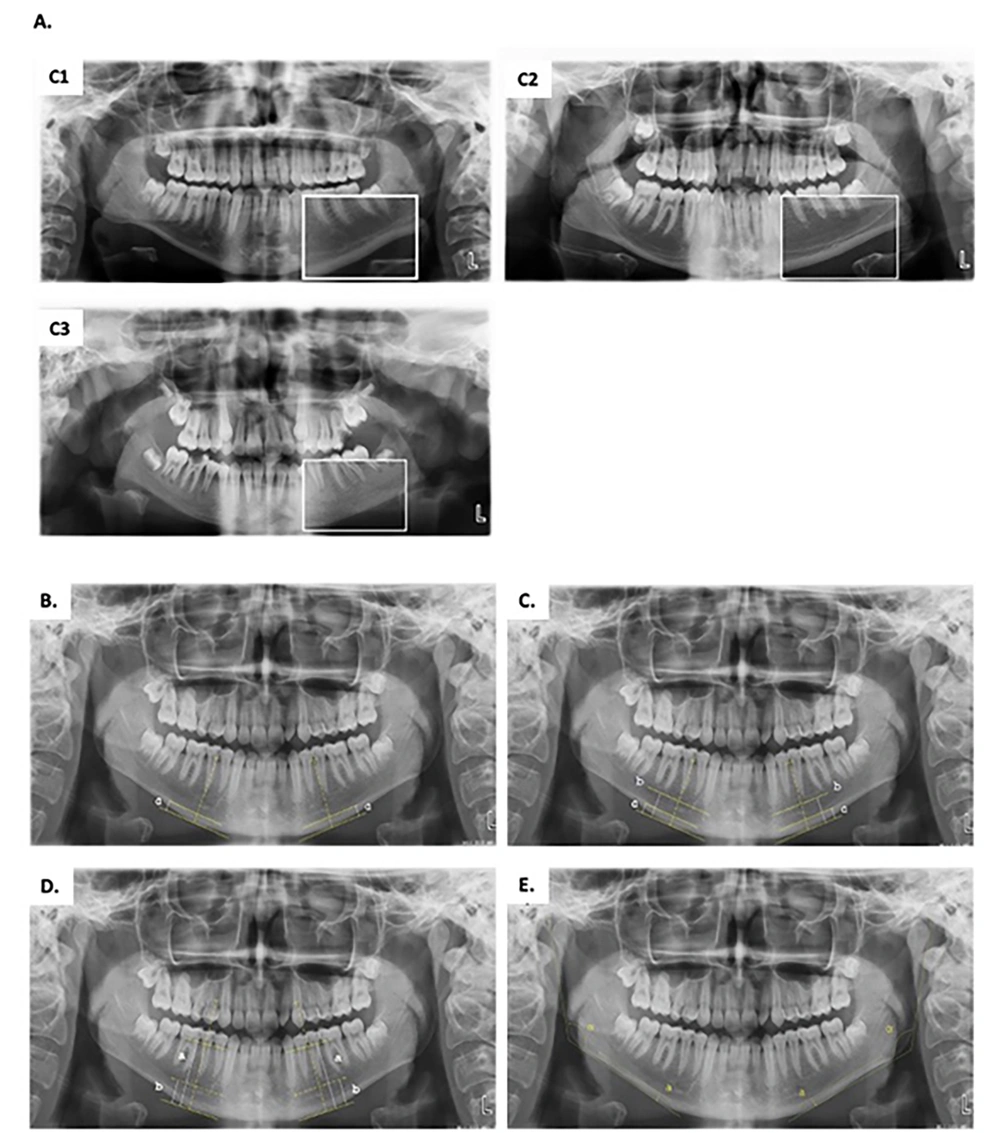
| Mandibular Cortical Index (MCI) | According to the classification by Klemetti and Kolmakow (10), the trabecular model of the cortical bone at the lower edge of the mandible was determined bilaterally and divided into 3 classes (Figure 2A): C1, Cortical bone being regular and continuous on the right and left sides; C2, Crescent-shaped defects in the cortical bone; C3, Severe irregularity in the cortical bone was classified as increased porosity. |
| Mandibular cortex width (MCW) | At the intersection of the line drawn tangentially to the lower edge of the mandible and the line passing through the midpoint of the mental foramen, the mandibular cortical width was measured on the right and left sides and averaged (Figure 2B). |
| Panoramic Mandibular Index (PMI) | The ratio of the width of the mandibular cortex to the distance between the foramen mentale and the inferior mandibular cortex was measured on both the right and left sides and averaged (Figure 2C). |
| Mental Index (MI) | The distance between the mental foramen and the inferior mandibular cortex was measured on the right and left sides and averaged (Figure 2C). |
| Alveolar bone resorption amount | The amount of alveolar bone resorption was calculated by the formula (2.9 x b)-a, according to the Wical technique (11) (Figure 2D). |
| Antegonial Index (AI) | The amount of cortical bone at the point where the antegonial depth of the line descending parallel to the ramus mandible and perpendicular to the corpus mandible from the junction of the ramus and corpus mandible is the widest, was measured separately on both sides, and the averages were taken (Figure 2E). |
| Gonial Index (GI) | The angle formed by the line drawn under the corpus mandible from the posteriormost point of the mandibular condyle to the back of the ramus and the mandible at the most posterior and lowest point was measured (Figure 2E). |
A, mandibular cortical index; C1, Normal bone cortex; C2, mild to moderately eroded cortex; C3, severely porous eroded cortex; B, mandibular cortical width (MCW) is indicated with a; C, mental index (MI) is indicated with b. The panoramic mandibular index (PMI) is calculated with the a/b ratio; D, the amount of alveolar bone resorption (AABR) was calculated by the formula (2.9 x b)-; E, Antegonial index (AI) is indicated with a. Gonial index (GI) is indicated α.
3.5. Statistical Analysis
SPSS 20 software was utilized. Data were expressed as numbers, percentages, means, medians, standard deviations, and minimum-maximum values. Normal distribution was assessed using the Shapiro-Wilk test. The Independent Samples t-test was applied when the normal distribution condition was met, while the Mann-Whitney U test was employed when it was not. The statistical significance level was set at P < 0.05.
Measurements of MI, PMI, MCI, ABRA, GI, and AI values were conducted by one researcher, MCW by another, and FD values by a third researcher. After 10 days, these values were reassessed on randomly selected 30 radiographs by the same investigators to evaluate intra-observer reliability. The intraviewer reliabilities were 0.930, 0.950, and 0.945 (Cohen’s kappa coefficient), respectively.
4. Results
Both the control and T1DM groups consisted of 21 women and 15 men, totaling 72 patients. While the mean age of the T1DM group was 12.31 ± 3.57, the mean age of the healthy group was also 12.31 ± 3.57. The BMI was 21.2 ± 2.1 for children with T1DM and 22.1 ± 1.2 for healthy children. There was no statistical difference between the groups regarding age and BMI (P = 0.441 and P = 0.321, respectively).
In the evaluation of MCI, the percentages of C1, C2, and C3 for the control group were 77.7% (28), 19.5% (7), and 2.8% (1), respectively, while in the T1DM group, the percentages were 75% (27), 22.2% (8), and 2.8% (1). There was no significance among the groups for MCI (P > 0.05).
While the average values of MI, PMI, MCW, ABRA, GI, and AI for the control group were 21.3 ± 4.1, 0.3 ± 0.07, 6.3 ± 1.8, 10.5 ± 8.0, 124.9 ± 5.5, and 5.2 ± 1.2, respectively; MI, PMI, MCW, ABRA, GI, and AI values for the T1DM patient group were 17.4 ± 6.8, 0.3 ± 0.05, 4.8 ± 1.8, 10.6 ± 7.2, 125.0 ± 5.3, and 3.9 ± 1.5, respectively. Mental index, MCW, and AI values were higher in the healthy children than in children with T1DM, and these differences were significant (P = 0.014, P = 0.001, and P < 0.001). There was no statistically significant difference among the groups for PMI, ABRA, and GI values (P > 0.05) (Table 2).
| T1DM | Non-diabetic | P-Value | Cohen d Effect Size | |||
|---|---|---|---|---|---|---|
| MI | 17.4 ± 6.8 | 15.7 (7.2 - 31.5) | 21.3 ± 4.1 | 21.0 (10.2 - 29.6) | 0.01 | 0.703 |
| PMI, mm | 0.3 ± 0.05 | 0.28 (0.19 - 0.45) | 0.30 ± 0.07 | 0.29 (0.17 - 0.46) | 0.28 | 0.259 |
| MCW, mm | 4.8 ± 1.8 | 4.8 (2.2 - 8.6) | 6.3 ± 1.8 | 6.2 (2.8 - 10.7) | 0.00 | 0.858 |
| GI, ° | 125.0° ± 5.3 | 125.0 (110.3 - 135.6) | 124.9° ± 5.5 | 125.1 (113.9 - 133.5) | 0.87 | 0.029 |
| AKRM, mm | 10.6 ± 7.2 | 9. (1.5 - 23.6) | 10.5 ± 8.1 | 9.7 (-4.4 - 28.9) | 0.99 | 0.016 |
| AI, mm | 3.9 ± 1.5 | 3.5 (1.9 - 6.7) | 5.2 ± 1.2 | 5.3 (2.3 - 7.4) | 0.00 | 0.988 |
a Values are expressed as mean ± SD or median (min-max).
While the average FD values in the mandible ramus, angulus, and corpus areas for the control group were 1.24 ± 0.09, 1.23 ± 0.10, and 1.23 ± 0.08, respectively, these values were 1.24 ± 0.07, 1.17 ± 0.08, and 1.18 ± 0.07 in the diabetic patient group. The FD values of the ramus area were not significant between the groups (P = 0.484). The T1DM patient group had lower FD values for the angulus and corpus regions, and there was a statistically significant difference (Table 3).
| T1DM | Non-diabetic | P-Value | Cohen d Effect Size | |||
|---|---|---|---|---|---|---|
| Ramus | 1.24 ± 0.07 | 1.25 (0.99 - 1.35) | 1.24 ± 0.09 | 1.25 (1.07 - 1.41) | 0.484 | 0231 |
| Angulus | 1.17 ± 0.08 | 1.17 (1.00 - 1.30) | 1.23 ± 0.10 | 1.26 (1.06 - 1.36) | 0.003 | 0.711 |
| Corpus | 1.18 ± 0.07 | 1.19 (1.00 - 1.30) | 1.23 ± 0.08 | 1.23 (1.10 - 1.40) | 0.029 | 0.543 |
a Values are expressed as mean ± SD or median (min-max).
5. Discussion
During growth, bone development is influenced by environmental and hormonal factors. Type-1 diabetes mellitus is often diagnosed during childhood or adolescence, which is a critical period for skeletal development. This study aimed to compare the cortical and trabecular structure of the mandible in pediatric patients diagnosed with T1DM and non-diabetic pediatric patients through radiomorphometric measurements and fractal analysis methods. Although there are studies on diabetes and bone structure, no similar study has been found in the literature.
It has been reported that osteoblastic activity is more active in children than in adults and gradually decreases with increasing age (12). In this study, a non-diabetic participant corresponding to the age of each participant in the T1DM group was selected to prevent age-related differences between the T1DM group and the healthy group in the evaluation of mandibular bone structure, and the mean age in our study was arranged similarly for both groups.
Panoramic radiography has advantages such as low-dose radiation and being a routinely used method (13). In our study, MI, MCW, AI radiomorphometric measurements, and FD values in the angulus and corpus regions were found to be higher in the healthy group than in the T1DM group. In clinical practice, it shows that easy-to-reach imaging techniques such as orthopantomography can facilitate the follow-up of growth and development in healthy children or children with systemic diseases, such as T1DM.
Fractal dimension values of the mandibular trabecular bone have been proven to be a good predictor of BMD in both men and women (14); however, it has been shown that while MCW is a good indicator for determining low BMD only for women (15). In this study, the same number of male and female participants in both groups was included to prevent gender-related differences between the T1DM and healthy groups.
It has been shown that MCI, used for the evaluation of cortical bone of the mandible, may be a sign of low BMD and remodeling in the mandible (14, 15). Although dual-energy X-ray absorptiometry (DEXA) is the reference evaluation method for osteoporosis, the MCI value may also have good sensitivity in determining osteoporosis (16). Mental Index can also be used to evaluate osteoporosis. It was claimed in previous studies that individuals with MI less than 3 mm have a higher predisposition to low BMD and should be referred for bone densitometry (17, 18). Another study claimed that bone thickness of less than 1 mm for the GI may be a sign of osteoporosis (19). Gulsahi et al. (20) proved that osteoporosis can be detected with adequate diagnostic efficiency in edentulous patients using MCW and MCI, but PMI doesn't have sufficient diagnostic efficiency. In a similar study conducted by Amorim et al. (21) and Dagistan and Bilge (18), a significant relationship in MCI was not observed between the control and osteoporosis patient groups, contrary to the findings of Gulsahi et al. (20).
Failure to control T1DM and delayed diagnosis can have a harmful effect on bone development and mineralization (11). In the study, a decrease in mandibular cortical bone was observed in children with T1DM compared to non-diabetic children, and this was associated with poor control of T1DM (8). Similarly, in our study, MI, MCW, and AI values were observed to be lower in the children with T1DM than in the healthy. In another study, MCW and PMI scores were found to be significantly lower when the children with T1DM were compared with the healthies (13). In our study, while the MCW value was observed to be higher in the healthy patients, there was no difference among the groups for the PMI value.
In a study (13), FD values of T1DM and Type-2 DM patients were compared with healthy individuals in specific regions of the mandible, and no difference was found among the groups. Contrary to this study, we found that while the FD values in the ramus region did not differ among the groups, the T1DM patient group had lower FD values in the corpus and angulus areas compared to healthy patients. This could be because our study was conducted on a pediatric patient group who were diagnosed for the first time and whose treatment has not yet started. In another study (22), it has been found that T1DM affects cortical thickness, fractal size, and bone microhardness, as well as microstructure and composition, and that insulin therapy reduces the effect of T1DM on cortical thickness and organic/mineral matrix. In this study and our study, it can be thought that T1DM affects bone metabolism and that bone metabolism returns to its normal state with the initiation of T1DM treatment.
Cortical bone is more affected by impaired remodeling mechanisms than trabecular bone; however, cancellous bone is a better indicator of metabolic activity and provides more valuable diagnostic information than compact bone because it is more metabolically active and has a higher bone turnover rate (15). Studies have revealed that nuclear factor-kappa-β (RANK), RANK ligand (RANKL), and osteoprotegerin (OPG) are found at higher levels in children with T1DM (23). These markers affect bone metabolism through osteoclasts and increase bone destruction. Our study supports this finding, and we can interpret that the decrease in fractal values and radiomorphometric indices in T1DM is due to osteoclast metabolism (24).
This study has some limitations. One of these is that the use of panoramic radiography is not compared with the CBCT system. Another limitation was that the changes in bone resorption markers in T1DM and healthy children could not be evaluated together with FD values and radiomorphometric indices.
In conclusion, our study examining the mandible in pediatric patients with T1DM using radiomorphometric measurements and fractal analysis method concludes that T1DM affects bone morphology and trabecular structure. According to the results of this study, the fractal analysis method and radiomorphometric measurements can be used in the clinical monitoring of bone development in patients with T1DM.
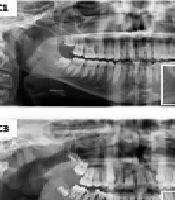
![A, selection of regions of interest (ROI) on panoramic radiography for use in fractal analysis (FA) [385 × 194 mm (120 × 120 DPI)]; B, fractal dimension (FD) analysis transactions; a, duplicated ROI; b, blurred image duplicated ROI; c, the blurred image was then subtracted from the original image; d, adding 128 to the result; e, application of 128 threshold value; f, erosion process; g, dilatation process; h, reversing; I, skeletonizing [237 × 119 mm (120 × 120 DPI)]; C, dividing image sizes into squares in specified pixels; D, inclination of the line showing the FB Value and analysis data for FB. A, selection of regions of interest (ROI) on panoramic radiography for use in fractal analysis (FA) [385 × 194 mm (120 × 120 DPI)]; B, fractal dimension (FD) analysis transactions; a, duplicated ROI; b, blurred image duplicated ROI; c, the blurred image was then subtracted from the original image; d, adding 128 to the result; e, application of 128 threshold value; f, erosion process; g, dilatation process; h, reversing; I, skeletonizing [237 × 119 mm (120 × 120 DPI)]; C, dividing image sizes into squares in specified pixels; D, inclination of the line showing the FB Value and analysis data for FB.](https://services.brieflands.com/cdn/serve/3170b/f8a4cca7a4d740eeef8934194de51abce1a31743/ijp-142061-i001-F1-preview.webp)