1. Background
At-risk infants are characterized by negative environmental and biological factors that occur during pregnancy, at birth, or after delivery, which contribute to the development of neurodevelopmental disorders and increased mortality. In Turkey, 2.80% of patients who apply to pediatric physiotherapy and rehabilitation units are considered at-risk infants (1). Various conditions, including inadequate prenatal care, congenital anomalies, premature birth, intrauterine growth restriction, periventricular leukomalacia, intraventricular hemorrhage, perinatal asphyxia, hypoxic-ischemic encephalopathy, chronic lung disease, and multiple births (twins or triplets), can elevate the likelihood of morbidity and mortality in both preterm and term infants who are at risk (2).
Furthermore, 11.1% of all births worldwide occur prematurely, and 5% of these births are at very low gestational age (< 28 weeks). Low and very low gestational ages, an immature central nervous system, and exposure to insufficient stimulation are risk factors for developmental problems, even in the absence of cerebral damage (1, 2). As gestational age decreases, more developmental problems arise, especially in terms of locomotor skills, hand-eye coordination, and motor performance (3). Neurodevelopmental disorders, cerebral palsy (CP), and visual and/or hearing impairment are the most common problems in preterm infants (1, 3-5). Therefore, neuromotor evaluations of preterm infants are important.
Infant neuromotor assessments serve diverse purposes, including differentiating infants with motor dysfunction from those with typical development (as a discriminative tool), predicting future motor issues based on current performance (as a predictive tool), and evaluating changes in motor abilities over time (6). Standardized instruments, tested using consistent scoring systems for validity and reproducibility, are used to detect early deviations in development and thus provide rapid referral of infants to interventional programs (7). Many methods, such as the Alberta Infant Motor Scale (AIMS), Hammersmith Infant Neurological Examination (HINE), and Standardized Infant Neurodevelopmental Assessment (SINDA) neurological scale, are used for neuromotor assessments of at-risk infants (8, 9). The AIMS is a norm-referenced test examining delays in motor performance of infants aged 0 - 18 months (8). The HINE is a measurement employed to assess the neurological development of infants ranging from 2 to 24 months of age (10). Those with high scores on the HINE performed at or before the corrected age of 2 years have better intelligence, verbal comprehension, perceptual reasoning, cognitive function, and neurological and motor functions in secondary school (11).
The SINDA neurological scale is a method consisting of five sub-parameters that evaluates spontaneous movements, cranial nerve functions, motor reactions, muscle tone, and reflexes of infants with corrected ages between 6 weeks and 12 months (9). The SINDA neurological scale is easy to apply and takes only 20 - 25 minutes, making it practical for clinical use. Evaluations are performed observationally without the use of any auxiliary devices, and the items are evaluated as passed or failed, simplifying its application (12). The SINDA neurological scale demonstrates reliable predictive validity for identifying atypical developmental outcomes in infants aged 24 months or older (12).
2. Objectives
The primary goal of this study was to assess the reliability and concurrent validity of the Turkish version of the SINDA neurological scale.
3. Methods
3.1. Research Design
This is a cross-sectional, multicenter study that included a test-retest component conducted to assess the reliability and validity of the Turkish version of the SINDA neurological scale from August 2020 to May 2021.
3.2. Participants
The study received approval from the ethics committee at Ondokuz Mayıs University (Approval Number: 41901325-050.99 2020/040). In adherence to the principles outlined in the Declaration of Helsinki, written informed consent was obtained from all participants’ parents. The sample size was calculated using G*Power (version 3.0.10; Franz Faul, Universität Kiel, Germany). One hundred and eleven at-risk infants (including 65 males and 46 females) were included in the test - retest reliability analysis with 98.8% power (13). Infants who had been admitted to the Pediatric Rehabilitation Unit were recruited for this methodological study. The departments in question were specialized tertiary outpatient clinics focused on infants who were at risk of or had been diagnosed with neurodevelopmental or neurological disorders. These infants were referred to the department by pediatric neurology practitioners for a range of reasons, including paroxysmal events, clinical indications of sensory deficits, unusual motor patterns (such as hypertonia, floppiness, and asymmetry), as well as physical findings like microcephaly and developmental delays. Furthermore, infants at risk of neurodevelopmental disorders, such as very preterm and newborn infants with neonatal complications, are followed by the department.
Infants were excluded if they had the following: (1) a known progressive neurological disorder (e.g., early-onset myotonic dystrophy, genetic refractory epileptic encephalopathy, refractory focal epilepsy, multiple side effects of antiepileptic drugs, structural West syndrome, and cortical malformation) and (2) congenital anomalies, musculoskeletal disorders, cyanotic congenital heart disease, and mechanical dependency.
3.3. Translation Process
Authorization for the Turkish translation was secured from Dr. Mijna Hadders-Algra, the senior and corresponding author of the original SINDA neurological scale, acting on behalf of all the developers of the scale (12). In accordance with prior research and established guidelines (14). both forward and backward translation methods were employed to carry out the Turkish adaptation of the SINDA neurological scale. Two independent professionals undertook the translation of the scale from English to Turkish. Following this, the translated versions were reconciled to form the initial version through discussions and consensus among the translators and the second author. Subsequently, a different bilingual translator, whose native language was English and who was unacquainted with the original SINDA neurological scale, conducted the back-translation, which was then reviewed by the original developers of the SINDA neurological scale. A committee of experts, including the first and second authors and two pediatric physiotherapists experienced in the field of children with disabilities, each with a minimum of 5 years of expertise, examined the ultimate Turkish rendition of the SINDA neurological scale.
3.4. Procedure
To gauge inter-rater reliability, two physiotherapists, each possessing over 5 years of experience in the field, evaluated the patient using the SINDA neurological scale on the same day. All tests were conducted according to standard protocols for each evaluation. Neither physiotherapist was aware of the other's assessment. For test-retest evaluation, one physiotherapist re-examined the patient one hour later on the same day. To assess concurrent validity, the HINE and AIMS were used on the same day, as described below. Patient characteristics (i.e., age, gender, and gestational age in weeks) were also recorded. The baseline assessment, which included rest intervals between tests to ensure the infant's physiological needs were met and their behavior was comfortable, took approximately 40 to 100 minutes to complete.
3.5. Measurements
3.5.1. SINDA Neurological Scale
The SINDA neurological scale demonstrates reliability and offers dependable predictive validity for identifying atypical developmental outcomes, such as cerebral palsy (CP), in at-risk infants with corrected ages ranging from 6 weeks to 12 months. Comprising 28 items, the scale places particular emphasis on assessing the quality of spontaneous motility, with a maximum achievable score of 28 points. The SINDA neurological scale comprises five distinct subdimensions: Spontaneous movements (eight items), cranial nerve function (seven items), motor reactions (five items), muscle tone (four items), and reflexes (four items). Each item is evaluated as a pass or fail based on clear and straightforward criteria. For several items, consistent asymmetry results in a 'fail' rating. Of the eight items in the spontaneous motility subdimension, seven assess the quality of movement in terms of variation versus stereotypy, while the eighth item evaluates the quantity of motility. The classification of movement variation versus stereotypy relies on clinical observation rather than video assessment, as is the case with the assessment of general movements. This evaluation can typically be completed in around 10 minutes. The intraclass correlation coefficients (ICCs) for both intra-rater and inter-rater agreement in the neurological score ranged from 0.923 to 0.965, indicating strong agreement. Item difficulty and discrimination were found to be satisfactory (9).
3.5.2. HINE
The Hammersmith Infant Neurological Examination (HINE) has recently been proposed as one of the early neurological examination tools for at-risk infants. It is a simple and scorable method designed for evaluating infants between 2 and 24 months of age. The HINE has been utilized across various populations, including high- and low-risk individuals, encompassing both preterm and term-born infants, and is used as a gold standard test in this study because of its neurological focus. It has been suggested as an alternative for prognosis, diagnosis, and rehabilitation purposes. The HINE consists of three main sections: The Neurological Examination, the Development of Motor Functions, and the State of Behavior.
The first section assesses cranial nerve function, posture, movements, muscle tone, and reflexes, with these items being age-independent. The second section focuses on evaluating head control, sitting, voluntary grasping, rolling, crawling, and walking. The third section pertains to the assessment of the infant's state of consciousness, emotional state, and social orientation. The data collected from the second and third sections are not included in the calculation of the global optimality scores; instead, they offer supplementary information for interpreting neurological findings. However, frequency distributions for these age-dependent sections were not computed.
The overall HINE score ranges from 0 to 78. The literature provides cut-off points at 3, 6, 9, and 12 months. For healthy term infants aged 3 and 6 months, the respective threshold scores for optimal performance are equal to or above 67 and 70 (median). The sensitivity (approximately 90%) and specificity of infants with a score of 56 or less at 3 months to predict the development of CP are high. At 9 or 12 months, scores equal to or greater than 73 are considered optimal, while scores below 73 are categorized as suboptimal. Babies with a score of 65 or less at 12 months have high sensitivity (approximately 90%) and specificity. Scores below 40 are only associated with severe CP.
3.5.3. AIMS
The Alberta Infant Motor Scale (AIMS) stands out as a valid and reliable assessment tool among those used to track changes in motor development and distinguish atypical motor behaviors in at-risk infants. Unlike conventional neurological examinations, this scale prioritizes functional abilities and the quality of movement, incorporating current normative reference values. It boasts high sensitivity, specificity, and accuracy in identifying motor deficits, making it suitable for monitoring motor development in infants during their first 18 months of life.
Gross motor development was evaluated using the AIMS, which is a norm-referenced observational tool designed to assess gross motor development in infants from birth up to the point of independent walking, typically around 18 months of age. The scale encompasses 58 items organized into four subscales: Supine (9 items), prone (21 items), sitting (12 items), and standing (16 items). These items are observed with regard to postural alignment, antigravity movements, and surface contact. The observed motor skills correspond to the infant's motor window, which includes all items falling between the less mature and more mature capabilities observed within the infant's motor repertoire.
Assessment was conducted through the unstructured observation of the child in different positions, such as prone, supine, sitting, and standing, based on the child's age. The total score on this scale can vary between 0 and 60 points. The resulting score can be converted into a normative age-dependent percentile rank, including the 5th, 10th, 25th, 50th, 75th, or 90th percentile. A score below the 10th percentile is indicative of possible delayed motor development (15, 16).
3.6. Data Analysis
All statistical analyses were performed using IBM SPSS Statistics Standard Concurrent User version 26 (IBM Corp., Armonk, New York, ABD) and Amos version 23 (Chicago: IBM SPSS) for confirmatory factor analysis. Descriptive statistics were used to describe infants’ demographic characteristics and assessment results. Continuous variables were expressed as mean (standard deviation), whereas categorical variables were reported as number (%). Internal consistency for the SINDA neurological scale was assessed by calculating and categorizing Cronbach’s alpha coefficient as follows: > 0.80 = excellent; 0.70 - 0.79 = adequate; and < 0.70 = inadequate. Intra-rater and inter-rater reliabilities were assessed using the ICC with the two-way random effects and absolute agreement methods. A principal component exploratory factor analysis was performed to investigate relationships between each dimension. We used the Kaiser criterion to retain any latent factors with eigenvalues equal to or greater than 1. Factor loadings of more than 0.5 were deemed highly relevant to the latent factor. Items with factor loadings above 0.40 were retained. The ratio of the chi-square test of model fit to the degrees of freedom (x2/df) (values of 5 or less), the Tucker Lewis Index (TLI: > 0.90 = acceptable and > 0.95 = excellent), the comparative fit index (CFI: > 0.90 = acceptable and > 0.95 = excellent), the standardized root mean square residual (SRMR: < 0.08 = acceptable and < 0.05 = excellent), and the root mean square error of approximation (RMSEA: < 0.08 = acceptable and < 0.05 = excellent) were used as goodness of fit statistics. To evaluate criterion validity, Spearman’s correlation coefficient was calculated between the scores of the SINDA neurological scale, HINE, and AIMS. The level of relationship was classified using Spearman’s correlation coefficient as follows: ‘< 0.30 = small/negligible’, ‘0.30 - 0.50 = low’, ‘0.50 - 0.69 = moderate’, ‘0.70 - 0.90 = high’, and ‘> 0.90 = very high’. The descriptive level of significance was set at P < 0.05.
4. Results
A total of 111 infants were included in this study. The sociodemographic information of the participants is given in Table 1. In line with these results, 41.40% of the children were girls, and 58.60% were boys. The mean corrected age of the infants was 15.36 ± 14.88 weeks, and the birth weight was 1818.60 ± 944.10 grams. Regarding the results of the risk examination, 64.86% of infants were high-risk, 31.53% of infants were medium-risk, and 4% of infants were low-risk. The risk level was determined by the criteria of the neurological score, which varied between 0.92 and 0.96, according to the Neonatal Society Guideline for High-Risk Infants for each infant (12).
| Characteristics | Values a |
|---|---|
| Gestational age (w) | 31.95 ± 4.27 |
| Weight at the birth (g) | 1818.60 ± 944.10 |
| Chronological age (w) | 23.07 ± 15.87 |
| Corrected age (w) | 15.36 ± 14.88 |
| Gender | |
| Female | 46 (41.40) |
| Male | 65 (58.60) |
| Level of risk | |
| High-risk infant | 72 (64.86) |
| Medium-risk infant | 35 (31.53) |
| Low-risk infant | 4 (3.60) |
| The Causes of risk | |
| Early preterm birth (< 32 w) | 49 (44.14) |
| Moderately preterm birth (32 - 34 w) | 34 (30.63) |
| Multiple birth | 3 (2.70) |
| Hypoxic ischemic encephalopathy | 14 (12.61) |
| Bronchopulmonary dysplasia | 3 (2.70) |
| Intraventricular hemorrhage | 4 (3.60) |
| Antenatal hemorrhage | 1 (0.90) |
| Large for gestational age | 2 (1.80) |
| Periventricular leucomalacia | 1 (0.90) |
Sociodemographic and Clinical Features of the At-risk Infants (n = 111)
4.1. Reliability of the SINDA Neurological Scale
The Cronbach’s alpha values for each subdimension in the SINDA are given in Table 2. As seen in Table 2, the factor loads of the subdimensions of SINDA were found to be sufficient because the Cronbach’s alpha (α) was above 0.70 (Cronbach’s alpha (α) = 0.81). Therefore, the six subsections of the SINDA measure separate features. The questionnaire we created according to these results is a reliable measurement tool. The total correlation values of the dimensions in our scale vary between 0.47 and 0.77, indicating that there is no need to make any reductions in the dimensions of the SINDA scale. Total correlation values should be above 0.40. If any of these correlations fall below 0.40, the corresponding item should be removed from the scale. To decide on the item to be removed, the "Item Subtracted Cronbach's Alpha" section of this part is examined. If removing the section increases the Cronbach's Alpha value, the relevant section should be removed from the scale. It is recommended not to remove any section if the total correlation value is above 0.40 and the Cronbach's Alpha value of the scale is above 0.70 (17).
| Variables | Factor Loads | Total Correlation | Item Subtracted Cronbach's Alpha |
|---|---|---|---|
| A1. Spontaneous Movements (Regional) | 0.73 | 0.54 | 0.79 |
| A2. Spontaneous Movements (General) | 0.84 | 0.74 | 0.77 |
| B.Cranial Nerves | 0.65 | 0.50 | 0.80 |
| C.Motor Reaction to Postural Stimulation | 0.61 | 0.47 | 0.81 |
| D.Muscle Tone | 0.87 | 0.77 | 0.74 |
| E.Reflexes and Reactions | 0.78 | 0.64 | 0.77 |
Explanatory Factor Analysis for the SINDA's Neurological Scale (n = 111)
4.2. Inter-Rater Reliability of the SINDA Neurological Scale
In Table 3, inter-rater reliability was evaluated for 6 sub-dimensions and 3 measurements for the total score obtained. As a result of these evaluations, measurements made at different times show high similarity in dimensions. Therefore, the Turkish version of the SINDA neurological scale has high inter-rater reliability.
| Variables | ICC | %95 CI | P-Values |
|---|---|---|---|
| A1. Spontaneous Movements (Regional) | 0.993 | (0.990 - 0.995) | 0.001 a |
| A2. Spontaneous Movements (General) | 0.995 | (0.993 - 0.996) | 0.001 a |
| B. Cranial Nerves | 0.997 | (0.996 - 0.998) | 0.001 a |
| C. Motor Reaction to Postural Stimulation | 0.994 | (0.991 - 0.996) | 0.001 a |
| D. Muscle Tone | 0.997 | (0.996 - 0.998) | 0.001 a |
| E. Reflexes and Reactions | 0.991 | (0.988 - 0.993) | 0.001 a |
| Total Score | 0.992 | (0.990 - 0.995) | 0.001 a |
Inter-Rater Reliability Analysis for the SINDA’s Neurological Scale (n = 111)
4.3. Validity of the SINDA Scale
4.3.1. Construct Validity
In Table 3, inter-rater reliability was evaluated for 6 sub-dimensions and 3 measurements for the total score obtained. As a result of these evaluations, measurements made at different times show high similarity in dimensions. Therefore, the Turkish version of the SINDA neurological scale has high inter-rater reliability.
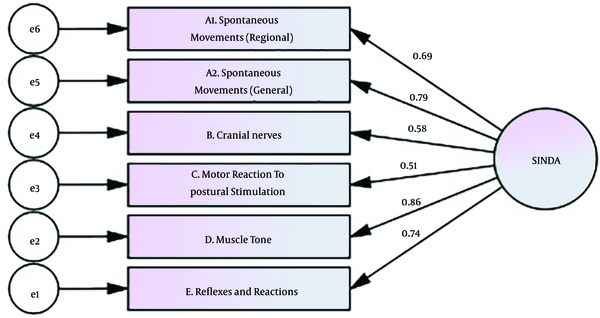
Construct validity of the SINDA was found to be acceptable (P < 0.05). The model fit of the scale is given in Table 4. In the model (χ2 = 22.44, df = 9, P < 0.001), there are four dimensions for the SINDA scale. When the fit indexes were examined in Table 4, χ2/sd = 2.60, RMSEA = 0.050, SRMR = 0.08, IFI = 0.947, CFI = 0.94, GFI = 0.93, and TLI = 0.90 were found. The KMO value was 0.82, and the Bartlett test result was 273.34 (P < 0.05). The obtained 57% variance showed that the questions significantly explained the SINDA scale. The tested model is shown in Figure 1.
Results of Confirmatory Factor Analysis
Table 5 shows the relationships for the 6 sub-dimensions and the total score. The level of relationship was classified using Spearman’s correlation coefficient as follows: ‘< 0.30 = small/negligible’, ‘0.30 - 0.50 = low’, ‘0.50 - 0.69 = moderate’, ‘0.70 - 0.90 = high’, and ‘> 0.90 = very high’. A correlation coefficient greater than 0.50 is considered acceptable for the correlation analysis. According to these results, the highest correlation between dimensions is between “Motor Reaction to Postural Stimulation” and the total score (r = 0.76, P = 0.001), and the lowest correlation is between “Spontaneous Movements (General)” and “Cranial Nerves” (r = 0.25, P = 0.006). The relationships between all subscores and the total score are statistically significant (P < 0.05).
Interdimensional Relationships for the SINDA's Neurological Scale (n = 111)
As shown in Table 6, each of the path coefficients in the SINDA sub-dimensions is statistically significant (P < 0.05). Accordingly, the highest effect on the total score was found in the “Muscle Tone” dimension (β = 0.86, P = 0.001), and the lowest effect was found in the “Motor Reaction to Postural Stimulation” dimension (β = 0.51, P = 0.001).
| Tested way | Standardized Estimate (β) | Estimate (β) | Standard Error | Critical Value | P-Values |
|---|---|---|---|---|---|
| E.Reflexes and Reactions ← SINDA | 0.74 | 1 | 0.001 a | ||
| D.Muscle Tone ← SINDA | 0.86 | 1.38 | 0.16 | 8.61 | 0.001 a |
| C.Motor Reaction to Postural Stimulation ← SINDA | 0.51 | 1.08 | 0.21 | 5.11 | 0.001 a |
| B.Cranial Nerves ← SINDA | 0.57 | 1.22 | 0.21 | 5.76 | 0.001 a |
| A1. Spontaneous Movements (Regional) ← SINDA | 0.78 | 0.83 | 0.10 | 7.96 | 0.001 a |
| A2. Spontaneous Movements (General) ← SINDA | 0.68 | 1.42 | 0.20 | 6.94 | 0.001 a |
Effects of Subdimensions on the SINDA's Neurological Scale
4.4. Concurrent Validity
The correlation between SINDA and AIMS and HINE was assessed for the concurrent validity study of the scale. The results of the correlations are given in Table 7. The concurrent validity of the SINDA was found to be acceptable. There were strong to weak correlations between SINDA subdomains and AIMS and HINE subdomains (r = 0.19 - 0.78; P < 0.05).
| Variables | A1. Spontaneous Movements (Regional) | A2. Spontaneous Movements (General) | B.Cranial Nerves | C.Motor Reaction to Postural Stimulation | D.Muscle Tone | E.Reflexes and Reactions | Total Score |
|---|---|---|---|---|---|---|---|
| HINE | |||||||
| Cranial Nerve Function | |||||||
| r | 0.31 b | 0.45 b | 0.58 b | 0.39 b | 0.36 b | 0.22 a | 0.56 b |
| p | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.019 | 0.001 |
| Posture | |||||||
| r | 0.38 b | 0.35 b | 0.30 b | 0.51 b | 0.39 b | 0.26 b | 0.55 b |
| p | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.005 | 0.001 |
| Movements | |||||||
| r | 0.47 b | 0.69 b | 0.46 b | 0.41 b | 0.59 b | 0.50 b | 0.66 b |
| p | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Tone | |||||||
| r | 0.30 b | 0.26 b | 0.43 b | 0.44 b | 0.42 b | 0.32 b | 0.53 b |
| p | 0.001 | 0.004 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Reflexes and reactions | |||||||
| r | 0.34 b | 0.34 b | 0.24 b | 0.55 b | 0.24 b | 0.06 | 0.50 b |
| p | 0.001 | 0.001 | 0.009 | 0.001 | 0.009 | 0.526 | 0.001 |
| Total Score | |||||||
| r | 0.49 b | 0.54 b | 0.52 b | 0.57 b | 0.52 b | 0.34 b | 0.72 b |
| p | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| AIMS | |||||||
| Total Score | |||||||
| r | 0.34 b | 0.40 b | 0.34 b | 0.78 b | 0.30 b | 0.23 a | 0.64 b |
| p | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.015 | 0.001 |
| Percentile | |||||||
| r | 0.05 | -0.01 | 0.19 a | -0.03 | 0.11 | 0.21 a | 0.08 |
| p | 0.556 | 0.848 | 0.045 | 0.730 | 0.243 | 0.021 | 0.356 |
Correlations Between SINDA’s Neurological Scale and HINE and AIMS Scales
5. Discussion
Neurodevelopmental evaluation of at-risk infants is important for their global development in follow-up clinics. The SINDA neurological scale is a valid and reliable tool to evaluate at-risk infants (9). This study is the first to investigate the psychometric properties of the Turkish version of the SINDA neurological scale for at-risk infants. Based on the findings of this study, the Turkish version of the SINDA neurological scale, which is a predictor of atypical neurodevelopmental outcomes among at-risk infants aged between 0 and 12 months, is a valid and reliable instrument.
Cronbach’s alpha values for each subdimension of the SINDA neurological scale are presented in Table 2. The factor loads of the subdimensions of the SINDA neurological scale were sufficient because the Cronbach’s alpha was above 0.70 (0.84). Therefore, the six subsections of the SINDA neurological scale measure separate features. The questionnaire we created according to these results is a reliable measurement tool. The total correlation values of the dimensions in our scale varied between 0.47 and 0.77, making any reductions in the dimensions of the SINDA neurological scale unnecessary.
The Kaiser-Meyer-Olkin (KMO) test assesses whether the distribution is adequate for factor analysis, and a range of 0.80 - 0.90 is considered excellent (18). Therefore, the KMO value in this study is at an excellent level. This measurement shows that the variable we examined is multivariate in the universe parameter. In this study, no limitation was placed on the number of factors, and factors with an eigenvalue equal to or greater than 1 were accepted as important factors (19). Considering that variance rates ranging from 40% to 60% are considered ideal in factor analysis, the amount of variance obtained in this study is at a sufficient level (20).
According to the results of our confirmatory factor analysis, the scale is suitable for Turkish culture. The results of our confirmatory factor analysis regarding construct validity support the six-factor structure of the original scale, and factor loadings were acceptable. The fit index values obtained showed that the model was in good agreement (21). The ratio of chi-square to degrees of freedom and fit indices is a method used to determine fit in cases where it is shown to be important in large samples (22). Confirmatory Factor Analysis is used to check whether the scale conforms to the original factor structure when used in the current research, and if so, to what extent. The results of the confirmatory factor analysis concerning structural validity support the original scale's six-factor structure. The fit index values obtained from confirmatory factor analysis have shown that the model is a good fit. In other words, each factor accurately represents the questions that comprise it. Our current confirmatory factor analysis results indicate that the scale is suitable for Turkish culture.
The SINDA neurological scale consists of six subsections and 28 questions. The results of the study showed that the scale had good inter-rater reliability. As a result of these evaluations, measurements made at different times show high similarity in dimensions. Therefore, the Turkish version of the SINDA neurological scale has high intra-rater and inter-rater reliability (22).
The ICCs were evaluated for the six subdimensions and three measurements for the total score obtained. These evaluations indicated high similarity in dimensions for measurements made at different times. To account for the growth and development of babies, the ICC was high because the evaluations were repeated within a short time period. As a result, the Turkish version of the SINDA neurological scale demonstrates high intra-rater and inter-rater reliability. Our results are similar to the intra-rater and inter-rater reliability values obtained in the original study of the scale (9).
In this study, the HINE and AIMS, known to be valid and reliable, were used to evaluate the concurrent validity of the Turkish version of the SINDA neurological scale. Early neuromotor or neurodevelopmental assessments should be valid, reliable, and capable of performing perinatal and early postnatal assessments. AIMS is one of the frequently used early neurodevelopmental test batteries with proven validity and reliability. It is stated that AIMS also evaluates the quality of movement and changes in motor skills (23). Studies (15, 24-26). have noted that AIMS is useful in determining neurological risk in infants in the early period. Liao and others (27). stated that AIMS is most sensitive in the evaluation of babies between 3 and 12 months. The HINE test, applied to determine the higher risk of neurological anomalies in preterm and term babies at other stages of their lives, is a preventive battery for detecting neural disorders in the early period (28). SINDA is also found to be a reliable and valid method as a screening tool in infancy. The correlation of the SINDA neurological scale with the HINE was r = 0.72, and its relationship with the AIMS was r = 0.64. These results show that the Turkish version of the SINDA neurological scale is valid.
Hadders-Algra’s original study reported 21 total SINDA neurological scale scores with 0.89 sensitivity and 0.93 specificity as the limit for atypical neurodevelopmental risk for children aged 24 months and older (9). Considering that 40.85% (n = 29) of the infants included in this study had a score of 21 and below, these infants have atypical neurodevelopmental risk and should be followed closely.
Infant neurodevelopmental assessments serve dual purposes. Initially, they focus on evaluating the current developmental state of the infant. Understanding the infant's present condition enables professionals to educate caregivers about their child's attributes and offer guidance on fostering their development, whether through professional early intervention or other means. Additionally, these assessments are utilized to anticipate and identify potential developmental disorders in at-risk populations (16, 29). In this study, SINDA’s neurological scale evaluated neuromotor development very well, paralleling other test batteries HINE and AIMS. These results show that SINDA’s neurological scale can be used in clinics for the early evaluation of at-risk infants, enabling earlier interventions and improved outcomes for infants in the Turkish population.
The limitation of this study was the lack of evaluation of the effects of clinical sociodemographic factors of the infants on neuromotor development. Interaction effects of gender, level of risk of the infants, and sociodemographic factors were not investigated, which might have affected the results. Further studies could determine if clinical properties and the sociodemographic factors of the infants affect neuromotor development as assessed with SINDA’s neurological scale.
5.1. Conclusions
Overall, this study demonstrated the reliability and discriminative validity of the Turkish version of the SINDA neurological scale. The Turkish version of the SINDA neurological scale is a valid and reliable tool, and we believe it will be a valuable asset in clinics as a fast and effective method for evaluating the neurodevelopment of at-risk infants in the Turkish population. The strengths of this study include the translation and adaptation process based on international guidelines and the inclusion of a large sample size with a wide age range. Furthermore, participants were recruited from several centers in different cities in Turkey, which might positively influence the generalizability of the scale.
In clinical settings, the Turkish version of the SINDA can be employed to assess the neurodevelopment of at-risk infants. This assessment provides insights into neuromotor outcomes, guiding professionals in determining suitable intervention methods and facilitating early intervention efforts.