1. Background
Classical Galactosemia (CG) is a rare, life-threatening, inherited autosomal recessive disorder that affects carbohydrate metabolism. In CG, the galactose-1-phosphate uridyltransferase (GALT) enzyme, a key enzyme in the Leloir pathway, is severely impaired, leading to significant health complications. Galactose-1-phosphate uridyltransferase plays a vital role in facilitating the conversion of galactose-1-phosphate (Gal-1-P) and uridine diphosphate glucose (UDP-glucose) into uridine diphosphate galactose (UDP-galactose) and glucose-1-phosphate (1). Disruption of these metabolic processes can trigger a severe disease affecting multiple organs, resulting in significant pathology and cognitive disability during childhood (2-4). In the general population, CG occurs in approximately 1 in 60,000 live births, with a slightly higher prevalence in the Caucasian population, affecting about 1 in 47,000 births (5, 6). The data on the prevalence of galactosemia in Iran is relatively limited; however, reports from our study area in Fars province suggest a prevalence of 5 in 24,000 (7, 8).
Newborn screening (NBS) programs for galactosemia during the first few hours of life vary among countries (9, 10). However, there is no universal consensus on galactosemia NBS, reflecting uncertainties regarding the balance between effectiveness, cost-effectiveness, and accuracy of existing screening methods (11). In 1964, a bacterial inhibition assay was introduced that could detect all cases of galactosemia, followed by the Beutler enzyme spot test in 1966, which detects only patients with GALT deficiency (12). Currently, there are other screening methods for galactosemia as well, the most common of which involve quantifying GALT enzyme activity or measuring total blood galactose (TGAL), which represents the sum of galactose and Gal-1-P on a dry blood spot (DBS) using a fluorometric assay (13). Multiple tests have since been developed, each with different rationales and mechanisms of action (microbiological, fluorometric, colorimetric, tandem mass spectrometric, and quantitative measurement of total galactose) (12, 14-17). However, a common practice among screening centers is to rely solely on the TGAL test as the initial screening tool, which is suitable for mass screening but carries a high rate of false-positive and false-negative results (18, 19). It is important to note that this test has shown a significant tendency to produce false-negative and false-positive outcomes (13).
To the best of our knowledge, high false positive rates occur significantly in different screening programs (19). A literature review by Varela-Lema et al. (20) highlighted the uncertainty regarding the wide variation in screening methods and the debate over different cut-off values (COVs). Additionally, it is worth mentioning that screening protocols for CG are influenced by regional variations in the incidence of CG (21, 22). High rates of false positive cases in NBS can have several significant long-term consequences. Positively labeled newborns must be recalled for clinical evaluation and further confirmatory laboratory tests, which can lead to excessive parental anxiety that may not fully resolve even after additional testing eliminates the possibility of illness, along with long-term consequences on the child–parent relationship (23, 24). Another ethical dilemma for both health workers and families is the interruption of breastfeeding for falsely labeled infants who are not at risk of negative consequences from milk consumption (22). Furthermore, a high number of unnecessary recalls and additional testing affect diagnosis and follow-up care for these infants, and the costs of galactosemia screening could considerably exceed the benefits, placing an additional financial burden on the healthcare system and patients (25, 26). Given the psychological and economic impact of false positives, it is crucial to continually refine screening methods to minimize false positives and improve the performance of NBS assays.
In Iran, based on our experience, using a TGAL cut-off of ≥ 4 mg/dL leads to a relatively good detection rate; however, it is accompanied by a fairly large number of false positive cases and recalls, resulting in unnecessary medical procedures, increased workload for healthcare employees, higher medical costs, and greater psychological effects (27). It is important to recalibrate current COVs to yield optimal sensitivity and specificity to ultimately minimize retesting and enable early diagnosis of definite cases requiring urgent therapy.
2. Objectives
Therefore, we conducted this study to evaluate the most predictive and optimal COVs for galactosemia screening based on TGAL levels in infants born between 2006 and 2020 in Fars Province, southwestern Iran. Our ultimate goal is to reduce false-positive and false-negative results and propose a unique protocol for our screening program.
3. Methods
All neonates, regardless of gestational age, birth weight, gender, hospital admission, or place of birth, born between August 2006 and December 2020 in Fars province, southwestern Iran, were enrolled in our study. The only exclusion criterion was neonates who died within 24 hours of birth. Data on patients, including TGAL levels, hospital admission, and the number of true positive cases used to assess the most effective cut-off value of the screening method, were provided by the Department of Non-communicable Diseases Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. All study protocols received approval from the Ethics Committee of Shiraz University of Medical Sciences under ethical code: IR.SUMS.MED.REC.1401.539. Moreover, the study was conducted in strict adherence to applicable guidelines, regulations, and the principles outlined in the Declaration of Helsinki. To ensure privacy and confidentiality, patient information was anonymized before analysis, and necessary measures were taken by the researcher to maintain data confidentiality. We evaluated patients with a positive screening test based on the manufacturer's kit guidelines. Heel prick samples were ideally collected between 24 and 72 hours after birth and were transported to a central laboratory at a tertiary care hospital in Shiraz with proper and timely shipment. All procedures were carried out in accordance with previous similar studies (11, 12, 14, 28). This means that a child with galactosemia born in Fars province, southwestern Iran, would have a diagnosis and begin treatment within the first week of life.
Total blood galactose testing was performed on DBS using S & S NO903 filter paper and the NEO-GAL Kit (Fardad Teb Arian Co., Tehran, Iran). Three dried blood spots (DBS) with a diameter of 5.0 mm were obtained from each control, standard, and sample to be analyzed. The DBS were incubated in glass tubes in a Benmari at 90 - 95°C for 10 minutes. This incubation took place in a reaction mixture of 150 µL, consisting of 50 mL Tris-buffer (170 mM, pH 8.0) and 50 mL G-1-P mix. The G-1-P mix contained 19.61 mL Tris-buffer (50 mM, pH 8.6), 24.51 mL NAD (0.013 M), 0.98 mL Gal-DH (2.5 mg/mL), and 4.9 mL alkaline phosphatase (300 U/mL). A 10 mL aliquot from each incubation mixture was carefully spotted onto filter paper and allowed to air dry at room temperature. The resulting dried spots were then subjected to visual interpretation under long-wave (490 nm) ultraviolet light. The intensity of the observed fluorescence correlated with the TGAL level.
Infants with their initial TGAL values ≥ 4 mg/dL were considered positive, and the respective treatment center for CG was informed by the screening laboratory. The parents were contacted and recalled by the treatment center for confirmation of the diagnosis. In confirmed cases, treatment was initiated with a restricted galactose diet, and the infants were routinely followed at the metabolic clinic. All diagnoses were confirmed either by genetic testing, considered the gold standard technique, or by the evaluation of the patient’s clinical features by a physician, based on the response to a galactose-restricted diet. Despite this, false-negative results on the initial screening test were encountered (14). False negatives associated with TGAL in this range were uncommon, and such patients, despite an initial negative test outcome, were likely to present clinically with features consistent with the disorder, such as liver dysfunction, susceptibility to infections, and failure to thrive. During follow-up evaluations, we identified instances of false-negative results. True positive cases of CG diagnosed through our screening process were those individuals who exhibited persistently elevated TGAL levels, along with typical symptoms of CG in the newborn period, such as jaundice, hepatomegaly, and feeding difficulties shortly after birth, who responded favorably to a lactose-restricted diet (29) (Table 1). At the time of the study, genetic testing was not performed for all cases due to the significant financial burden, which aligns with other studies that did not use genetic methods (14, 19).
| Characteristic | True Positive | False Positive |
|---|---|---|
| Enzyme activity | Significantly reduced or absent GALT enzyme activity (19) | Normal or near-normal GALT enzyme activity |
| Biochemical markers | Persistent elevation of galactose-1-phosphate in red blood cells (30) | Normalization of biochemical markers upon follow-up |
| Screening results | Elevated levels of TGAL (galactose-1-phosphate and total blood galactose) (11) | Elevated levels due to other conditions or sample handling issues |
| Genetic confirmation | Confirmed by genetic analysis showing mutations in the GALT gene (31) | No mutations in the GALT gene |
| Clinical symptoms | Jaundice, hepatomegaly, feeding difficulties, failure to thrive (32) | Typically, asymptomatic or symptoms due to other conditions |
| Follow-up testing | Consistently abnormal results in follow-up tests (11) | Normal results in follow-up tests |
| Management | Requires dietary interventions and ongoing monitoring (33) | No specific treatment required for galactosemia |
| Long-term outcomes | Risk of developmental delays and other complications if not managed properly (34) | Generally good prognosis if false positive is identified and managed appropriately |
| Family history | Often a family history of galactosemia or related metabolic disorders (35) | Typically, no family history of galactosemia |
We conducted statistical analyses using SPSS Version 26.0. First, we assessed the normal distribution of TGAL levels using the Kolmogorov-Smirnov test. Next, we used the Mann-Whitney test to examine associations between factors. The descriptive results are presented as frequencies and percentages (%) or as medians and interquartile ranges (IQR). To assess the sensitivity and specificity of TGAL at various COVs, a receiver operating characteristic (ROC) curve analysis was performed. A ROC curve is a two-dimensional graphical plot that demonstrates how well a classifier system performs by varying the discrimination cut-off value across the range of the predictor variable (36). The ROC curve provides sensitivity and specificity calculated at different cut-off values, with an optimal cut-off value representing the best performance of the classifier. The area under the curve (AUC) was used as a measure to assess the predictive accuracy of TGAL in identifying high-risk neonates for galactosemia. A P-value of less than 0.05 was considered statistically significant.
4. Results
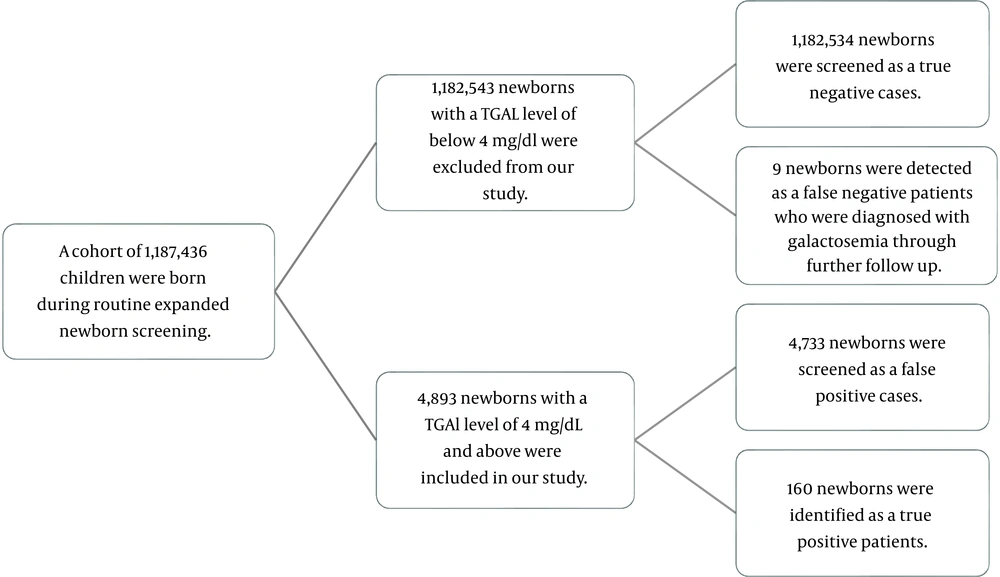
A total of 1,187,436 newborns were screened in our database from August 2006 to December 2020 (15 years), representing the total birth population in our province during this timeframe. The population consisted of 616,950 (51.96%) male and 570,486 (48.04%) female neonates. A TGAL level of ≥ 4 mg/dL was found in 4,893 (0.41%) newborns, who were presumed to have a positive test on initial screening, while the remaining 1,182,543 neonates were labeled as negative screening test cases.
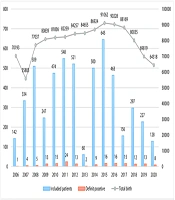
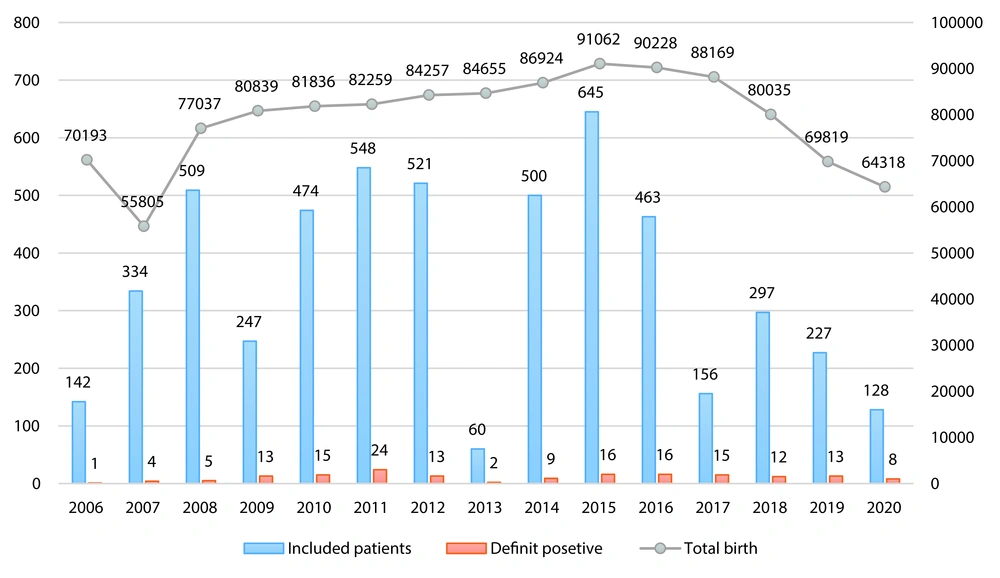
Among the 4,893 positive screened patients, 2,481 (50.7%) were male and 2,412 (49.3%) were female. Based on clinical features and DNA analysis identified by NBS, 160 (3.26%) neonates were confirmed as true positive cases of CG, while 4,733 (96.7%) were false positive cases. Additionally, during follow-ups, nine newborns who were initially labeled as negative results were later confirmed as positive for CG, representing our false negative cases (Figure 1). Our data demonstrated a total prevalence of 0.01% for CG in our province. Figure 2 illustrates the prevalence of confirmed galactosemia newborns among the total annual births and newborns with a TGAL level of ≥ 4 mg/dL. The mean annual incidence rate was 13.7 per 100,000 (range: 1.4 - 29.2) during 2006 – 2020 in Fars province, with the highest incidence observed in 2011.
The mean TGAL measured was 6.34 mg/dL (median: 4.1; Q1 - Q3: 4 - 5). Additionally, 30 (18.75%) known or suspected galactosemia cases had a history of hospital admission. Based on the Mann-Whitney test, hospitalized patients had significantly higher TGAL scores compared to non-hospitalized patients (median: 53.75 vs. 4.10; P < 0.001).
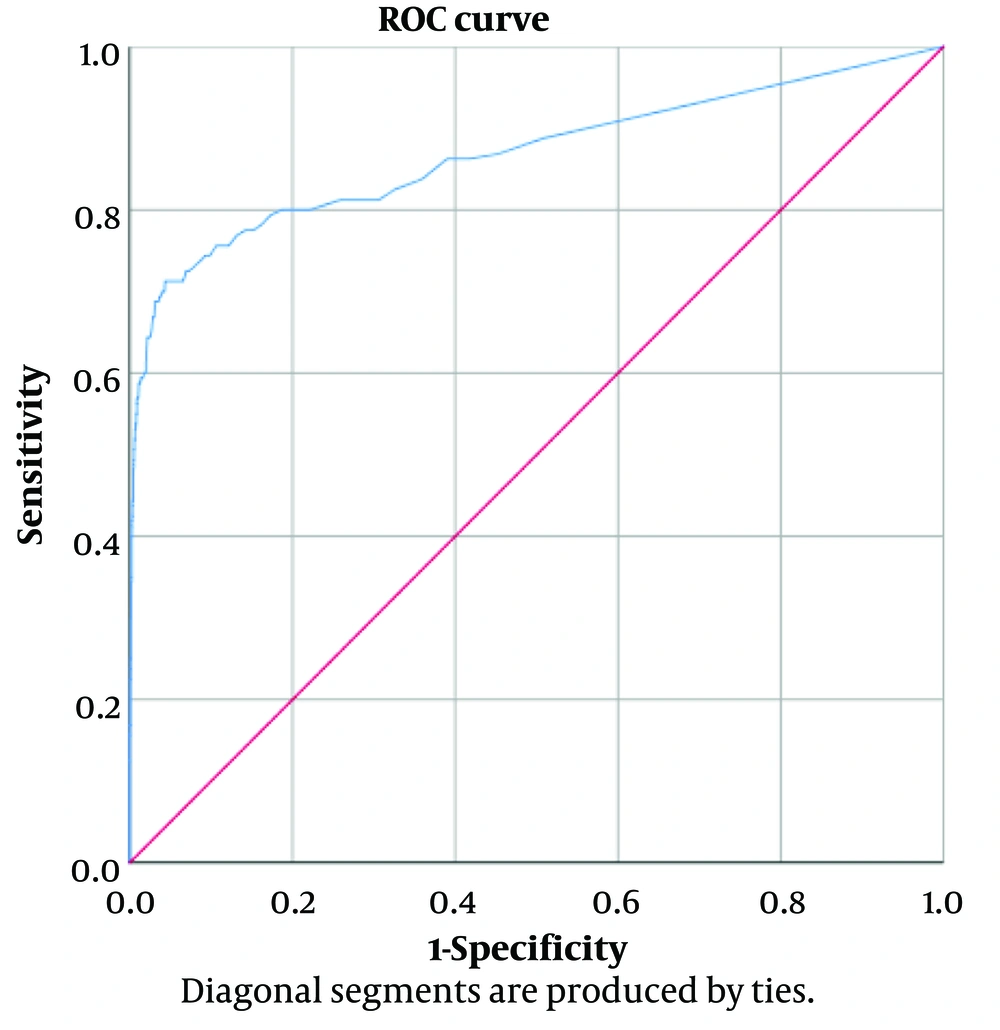
Receiver operating characteristic curve analysis revealed that a COV of 7.35 mg/dL for TGAL was the most predictive for CG, with the highest cumulative sensitivity of 71.3% and specificity of 95.7%. The curve and the corresponding AUC of 0.868 demonstrated TGAL as a biomarker with strong predictive ability to discriminate galactosemia from normal subjects (Figure 3). Furthermore, we estimated that setting a cut-off level of 5.2 mg/dL for TGAL could yield a sensitivity of 80.0% and a specificity of 81.3% for confirming true positive cases (Table 2).
| Variables | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Cut-off 7.35 (mg/dL) | 71.3 | 95.7 | 100 | 27.94 | 99.98 |
| Cut-off 5.20 (mg/dL) | 80.0 | 81.3 | 100 | 8.54 | 99.92 |
| Cut-off 4.00 (mg/dL) | 88.8 | 49.2 | 100 | 2.32 | 99.60 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
5. Discussion
This paper explores the appropriate COVs and determines their validity (sensitivity and specificity). These results further highlight a significant increase in the accuracy of the proposed COVs as an alternative to the current cut-off. Herein, we introduced a new protocol for NBS screening. A review of all cases with TGAL ≥ 4 mg/dL provides the following observations: Based on either DNA or clinical confirmation, CG was confirmed in 169 neonates, with 160 true positive cases and nine false negative results. The TGAL level appears to be a reliable predictor for distinguishing individuals with CG. Our ROC analysis revealed a sensitivity of 88.8% and a specificity of 49.2% for the current cut-off (TGAL ≥ 4 mg/dL), with a positive predictive value of 2.32. The low specificity of the 4 mg/dL COV has cost implications due to the high recall rate.
To minimize high false positive rates, the cut-off is constantly adjusted to improve specificity without compromising sensitivity. Similarly, Freer et al. proposed a TGAL COV of 30 mg/dL for urgent reporting, based on assessing the combination of TGAL levels and GALT activity (14). However, highly specialized instruments are usually required for these techniques. Additionally, quantifying GALT activity involves complicated sample processing, which is influenced by high temperature, humidity, and the duration between sampling and testing. Furthermore, Porta et al. (30) established a COV of 10 mg/dL in a population of 1,123,909 patients, resulting in 8,991 recalls and 33 abnormal results, indicating a high rate of false positives in the program. Similarly, Fujimoto et al. (17) proposed a COV of 7 mg/dL, incorporating enzyme extraction and reaction in their screening program. They also recommended measuring GALT activity as a second-tier test to help distinguish between various forms of galactosemia. This method is not simple or practical, as it requires a Technicon auto analyzer with a special fluorometer. However, these efforts must be feasible and financially accountable for NBS programs.
The primary goal of NBS is to proactively detect disorders and implement a tailored management strategy before symptoms manifest, thereby minimizing potential harm. With a COV of TGAL ≥ 5.2 mg/dL, the screening achieves a conservative approach with 80.0% sensitivity, 81.3% specificity, and a positive predictive value (PPV) of 8.54%. This threshold effectively reduces false positives and recalls compared to the current COV of 4 mg/dL. While the latter is effective at excluding most cases without oversight, the former strikes a balance by enhancing detection accuracy for GALT-deficient neonates. Moreover, the COV of TGAL ≥ 5.2 mg/dL, compared to the COV of 7.35 mg/dL, prevents a higher number of false negative cases. Following a positive NBS test, the newborn is referred to a specialized clinical center where their clinical features are evaluated. Diagnostic confirmation is then performed through biochemical and molecular methods.
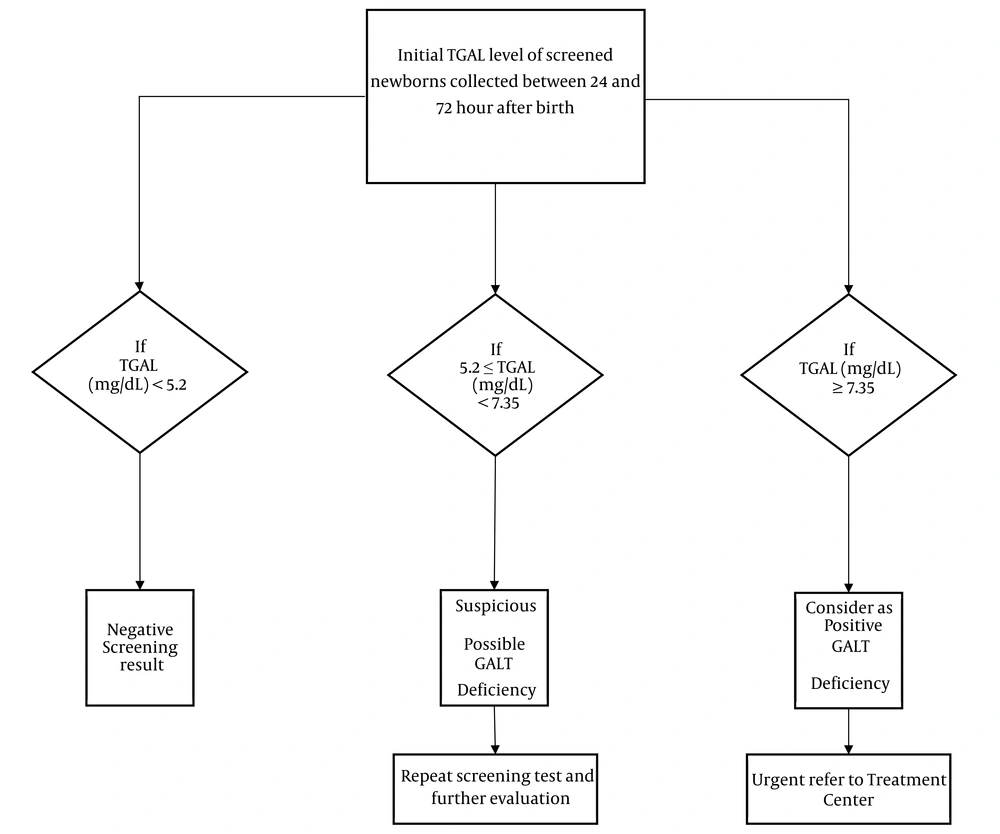
In our study, it was found that the vast majority (96.7%) of individuals who tested positive for galactosemia in the screening were not deficient in GALT. Therefore, we developed a flow diagram to reflect our suggested COVs (Figure 4). A unique COV of 7.35 mg/dL for TGAL, with the highest cumulative sensitivity of 71.3% and specificity of 95.7%, along with a relative PPV of 27.94%, was identified as the optimal COV. Our proposed revisions to clinical protocols suggest that patients with a TGAL level of 7.35 mg/dL or higher, demonstrating a specificity of 95.7%, should be classified as candidates for urgent calls. These patients should be promptly referred to a galactosemia treatment center for full dietary restriction and appropriate confirmatory testing. We propose that a TGAL value < 5.2 mg/dL can be considered a negative screening result, and a TGAL value between 5.2 mg/dL and 7.35 mg/dL should be reported as inconclusive, requiring a repeat screening test as soon as possible.
One of the key principles of our proposed screening is the reduction of the false positive rate, which would be cost-effective and outweigh the psychological harm caused by the test. A study assessing the cost-effectiveness of including galactosemia in the NBS program in Shiraz stated that the financial burden of galactosemia was reduced by two-thirds through the introduction of neonatal screening for galactosemia (10). It is also important to note that the anxiety experienced by parents or relatives of screen-positive newborns until the confirmation of results is primarily derived from false positives. In light of the current findings, we believe that increasing the TGAL cut-off to 5.2 ≤ TGAL (mg/dL) < 7.35 could reduce false positives to acceptable levels.
Our study has some limitations. Notably, not all reported cases of galactosemia were confirmed with DNA exome sequencing due to significant financial constraints. Additionally, the original data collection commenced in 2006, which led to incomplete access to patients' documents for follow-up laboratory data assessment. Further studies are essential to evaluate the efficacy of the suggested policy for NBS in reducing false positives. It is important to note that given the constraints on available resources, we employed this method to ensure the feasibility and integrity of our research. Future screening methods should focus on being well-established, inexpensive, and not demand a heavy workload.
5.1. Conclusions
In conclusion, our study indicated the prevalence of CG to be 1: 10,000 in neonates. We identified a TGAL level of 5.2 mg/dL as a conservative cut-off for CG screening, demonstrating excellent sensitivity and ensuring specificity for recalling suspicious cases. Additionally, a cut-off of 7.35 mg/dL was proposed for cases requiring urgent treatment. This represents the cut-off point at which the combination of sensitivity and specificity is maximal, making it the "optimal" cut-off point. The accuracy of the proposed COVs is substantially higher than the current cut-off. Implementing an appropriate cut-off value not only saves money and time but also reduces stress on an already overburdened system. Further clinical studies and practical application are necessary to support our findings.