1. Background
Progressive familial intrahepatic cholestasis (PFIC) is a diverse group of liver disorders that impair bile acid formation or secretion and lead to hepatocellular cholestasis in childhood. Although it is an autosomal recessive inheritance, the parents of these children are often asymptomatic, and the diagnosis can be missed (1, 2). Both genders are equally affected by PFIC. Pruritus and jaundice are major clinical presentations of PFIC. Progressive familial intrahepatic cholestasis has fatal complications, including portal hypertension, hepatic fibrosis, cirrhosis, liver failure, and hepatocellular carcinoma (HCC).
Progressive familial intrahepatic cholestasis diagnosis is based on a detailed history, physical examination, laboratory findings, radiologic evaluations, histological evaluations, and genetic study (2-4). Therapeutic strategies for PFIC patients include medications, dietary supplements, and surgical interventions, such as internal or external biliary diversions and liver transplantation for patients with progressive liver fibrosis and end-stage liver disease and for patients with severe intractable pruritus (2, 5). However, there are challenges in the diagnosis and treatment of PFIC for physicians; therefore, finding a disease biomarker might be of great importance.
MicroRNAs are small, single-stranded, noncoding RNAs (6). The miR-34 family includes three members, miR-34a, miR-34b, and miR-34c, which are differentially expressed in mice and humans. Earlier studies have demonstrated multiple functions and targets of the miR-34 family. microRNA-34a (miR-34), a member of miR-34 family, is located on chromosome 1p36 and is a direct target of tumors suppressor p53. It is implicated in tumor suppression via inhibiting proliferation, epithelial to mesenchymal transition, metastasis, and stemless, as well as enhancing apoptosis, cell cycle arrest, and senescence. Additionally, miR-34a has important roles in various biological processes, such as aging, neuronal development, and stem cell differentiation (6-9).
The miR-34 family stimulates hepatic stellate cells that result in the development of hepatic fibrosis. Additionally, miR-34 is involved in different liver disorders, such as liver fibrosis, non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease, and HCC (10-13). Previous studies reported increased levels of mir-34a in NAFLD and its positive association with disease severity, inflammation, fibrosis stage, and disease progression (14, 15). Tian et al. showed that the activation of miR-34a/SIRT1/p53 signaling pathway induces apoptosis in hepatocytes, thereby promoting liver fibrosis; therefore, it might be a promising therapeutic target for liver fibrosis (16). This pathway is also linked to NAFLD severity (17, 18).
Moreover, miR-34a enhances epithelial-mesenchymal transition (EMT) and liver fibrosis through TGF-β1/Smad pathway and might serve as a biomarker for liver fibrosis (13). miR-34a is also increased after long-term alcohol consumption and regulates cell proliferation, migration, and remodeling in alcoholic liver injury by targeting caspase-2 (CASP2) and SIRT1 and upregulating matrix metalloproteinase 2 and 9 (MMP2 and MMP9) expression (10, 11).
Although there are several studies regarding miR-34 roles in liver diseases, few investigations have been performed on miR-34 expression in PFIC patients. Since it is essential to find non-invasive methods for determining PFIC diagnosis and prognosis, this study evaluated the expression of miR-34a in PFIC patients.
2. Objectives
This study aimed to evaluate the expression of miR-34a in PFIC patients.
3. Methods
3.1. Patients
In this cross-sectional study, 18 PFIC patients and 18 healthy subjects were included. Progressive familial intrahepatic cholestasis patients were randomly selected from the Shiraz Pediatric Liver Cirrhosis Cohort Study (SPLCCS) (19, 20). Laboratory and clinical information of children were extracted from the pediatric liver cirrhosis registry (IR.SUMS.REC.1399.530). The diagnosis of PFIC is based on clinical signs and symptoms, routine liver tests, radiographic examinations, and liver biopsy.
The blood samples were stored in the laboratory of Shiraz Transplant Research Center at -80°C. The control group consisted of 18 children referred to surgical wards for minor surgeries, such as tonsillectomy, with no history of liver diseases. Additionally, the parents were asked about their liver disease history when filling out the personal information forms of children. All members of the control group and their parents had no history of liver disease.
The written informed consent was obtained from each participant’s parents. This study was approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (IR.SUMS.MED.REC.1401.462)
3.2. microRNAs Extraction and Quantitative Polymerase Chain Reaction
miRNA was extracted from the serum samples of PFIC patients using RNX-Plus (Cinnagen, Iran) according to the manufacturer’s instructions. The cDNA was synthesized using an RB-Micro RNA Synthesis kit (RNA biotechnology company, Iran) by specific stem-loop primers using 300 ng total RNA in a 20-μL total volume. The reaction included 10 mM dNTP, 100 pMol RT primer, 100 pMol stem-loop primer for MircoRNA, 5x reverse transcriptase buffer, and M-MLV reverse transcriptase enzyme. This process was performed in a thermal cycler (ABI) under the following conditions: 60 °C for 10 minutes, 55°C for 40 minutes, 72°C for 15 minutes, and 12°C as holding.
Real-time polymerase chain reaction (PCR) was performed using the Step-One ABI applied Biosystem (Life Technologies). U6 snRNA was used as an internal control for the analysis of miR34 expression. The reactions were carried out by the following parameters: 95°C for 15 minutes, 95°C for 15 seconds, and 62°C for 60 seconds for 40 cycles. Each sample was measured three times, and mean cycle threshold (Ct) values of measurements were used for the following analysis. To determine miR34 expression in the sample, the Livak (2−ΔΔCt) method was employed.
3.3. Statistical Analysis
For continuous and categorical variables, data were presented as mean ± standard deviation (SD) or number (%). Data normality was checked by the Shapiro-Wilk test and skewness and kurtosis indices. To compare the two groups, the Student’s t-test (or Mann-Whitney test) and the Chi-square tests were used. Additionally, the relation of miR-34 expression with laboratory and baseline data and gender in the cases was analyzed using Spearman’s test and the Mann-Whitney test. The data analysis was conducted by SPSS software (version 16. Chicago, SPSS Inc., United States), and a p-value < 0.05 was considered statistically significant.
4. Results
4.1. Demographic and Clinical Characteristics of the Case and Control Groups
The demographics and laboratory data from the study groups are shown in Table 1. A total of 18 PFIC patients and 18 healthy individuals were enrolled in this study. The mean age of the control group was 5.8 ± 2.25 years, and 50% of the participants were female. Among the 18 PFIC patients, 33.3% were female, with a mean age of 2.95 ± 3 years. Progressive familial intrahepatic cholestasis patients had considerably higher liver enzyme levels, including aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALKP), than the control group. The detailed features of the PFIC group, their family, and surgical history are presented in Table 2.
| Variables | PFIC Group (n = 18) | Control Group (n = 18) | P-Value |
|---|---|---|---|
| Gender | 0.310 | ||
| Male | 12 (66.7) | 9 (50) | |
| Female | 6 (33.3) | 9 (50) | |
| Age, y | 2.95 ± 3.02 | 5.81 ± 2.25 | 0.003 |
| AST, U/L | 369.50 ± 1005.76 | 23.22 ± 7.88 | < 0.001 |
| ALT, U/L | 201.22 ± 438.85 | 8.38 ± 3.97 | < 0.001 |
| ALKP, U/L | 742.33 ± 418.81 | 429.44 ± 136.28 | < 0.001 |
| Total protein, g/dL | 6.11 ± 1.39 | 5.85 ± 0.81 | 0.708 |
| Albumin, g/dL | 3.82 ± 0.57 | 3.90 ± 0.43 | 0.605 |
| Total bilirubin, mg/dL | 8.06 ± 11.17 | 0.44 ± 0.07 | < 0.001 |
| Direct bilirubin, mg/dL | 5.45 ± 8.32 | 0.18 ± 0.08 | < 0.001 |
| WBC count, per 1000 | 12.40 ± 9.17 | 7.72 ± 1.75 | 0.003 |
| Hb, g/dL | 10.69 ± 1.97 | 13.03 ± 1.06 | < 0.001 |
| Platelet count, per 1000 | 251.05 ± 134.50 | 327.66 ± 53.24 | 0.021 |
a Data were presented as mean ± SD (range) for continuous and No. (%) for categorical variables, respectively.
| Variables and Characteristics | Frequency (%) |
|---|---|
| Parents relation | |
| First degree relative | 12 (66.7) |
| Second degree relative | 4 (22.2) |
| Unrelated | 2 (11.1) |
| Family history of liver diseases | |
| Positive | 5 (27.8) |
| Negative | 13 (72.2) |
| Liver transplantation | 10 (58.8) |
| Surgical history | |
| GI-related | 6 (33.3) |
| Non-GI-related | 1 (5.6) |
| Death | 4 (22.2) |
4.2. Increased miR-34a Expression in PFIC Patients in Comparison to the Control Group
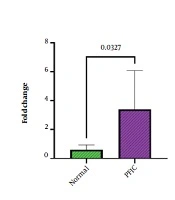
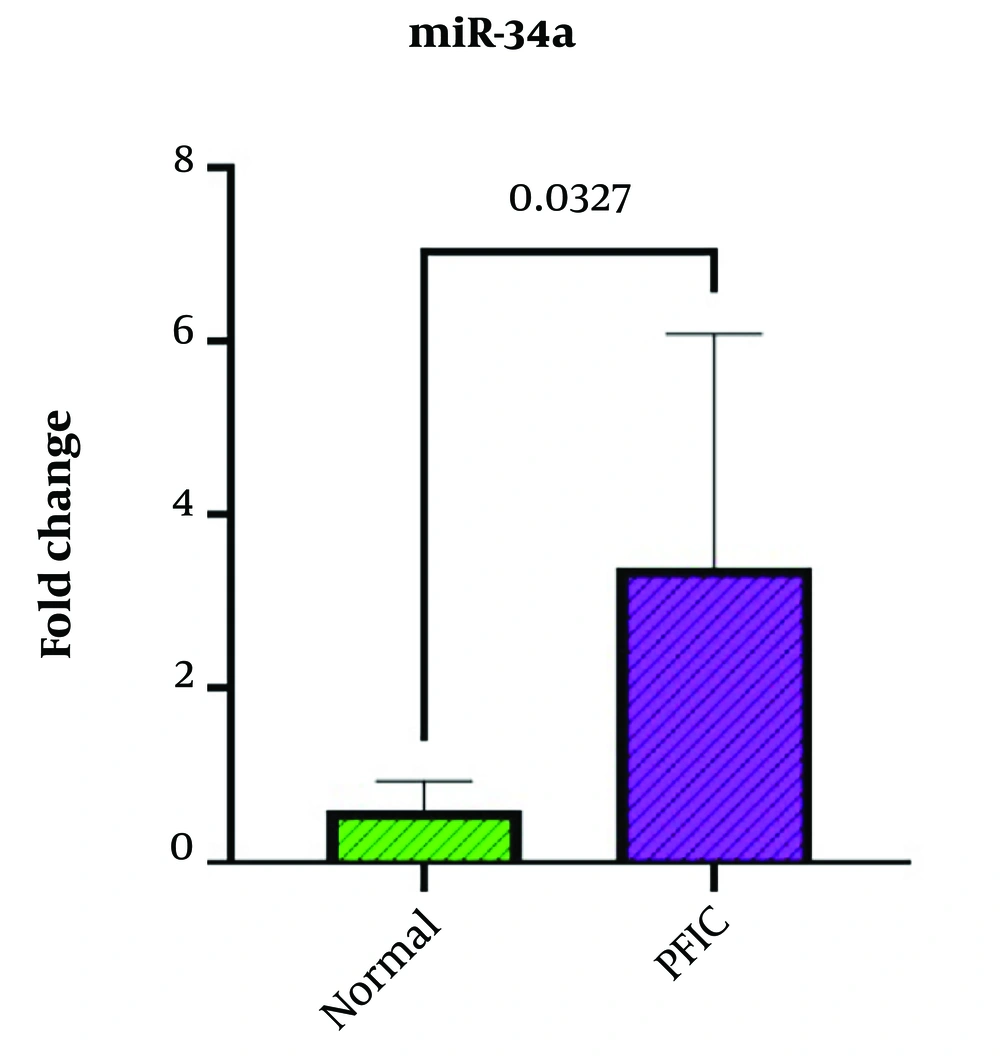
We analyzed the expression of miR-34a in the serum of the case and control groups and investigated the differences between miR-34a expression levels among these two groups. The obtained results revealed that miR-34a plasma levels were considerably elevated in PFIC patients (mean fold change = 3.39) in comparison to healthy subjects (mean fold change = 0.6) with a p-value of 0.0327 which was considered statistically significant (Figure 1).
5. Discussion
Pediatric liver disease usually has nonspecific symptoms during the early stages of the disease, leading to delayed diagnosis. Progressive familial intrahepatic cholestasis diagnosis is based on a detailed history, physical examination, laboratory findings, radiologic evaluations, histological evaluations, and genetic study. Although miRNAs are tissue- and disease-specific, they might be found in body fluids and remain stable in circulation. This issue makes them interesting targets to be utilized as diagnostic and therapeutic biomarkers (21-24).
miRNAs are implicated in a variety of biological processes, such as organ development and physiology, in addition to cell differentiation, proliferation, and cell cycle modulation in all types of cells, including the hepatocytes (11, 21, 25). miR-34a, a direct target of p53, plays an important role in cell proliferation and apoptosis, and its association with several liver disorders, such as NAFLD, alcoholic liver injury, liver fibrosis, and HCC, has been reported previously (10, 12, 13). An experimental study on mice with cholestatic liver disease showed that miR-34a plays an important role in ductular reaction and fibrotic responses (26).
Considering the above-mentioned studies and miR-34a roles in various liver disorders, it was decided to evaluate the expression of miR-34a in PFIC patients and compare it to a healthy control group in this study. The results of the current study showed that the serum levels of miR-34a were significantly elevated in comparison to healthy subjects. These results are consistent with those of a study performed by Rieger et al., which reported significantly higher miR-34a expression in patients with cholestasis than in those without cholestasis and confirmed the association of miR-34a with cholestatic liver diseases (27). Li et al. conducted a study on serum samples from patients with chronic hepatitis C, revealing that miR-34a expression was comparative to serum levels of total bile acid (TBA), AST, and ALT. They concluded that miR-34a promotes liver fibrosis and might function as a prognostic biomarker for chronic hepatitis C (28).
In this study, however, no correlation was observed between miR-34a expression levels in PFIC patients and serum aminotransferases (ALT and AST), ALKP, total protein, albumin, and bilirubin. Muangpaisarn et al. also reported no significant correlation between miR-34a and clinical factors, such as age, weight, and height in NAFLD patients; they indicated a fair, positive correlation between serum levels of miR-34a and ALT (r = 0.42, P = 0.002) (14). One explanation is that patients with severe liver disease receive albumin substitution, which overestimates the measured protein serum levels. Additionally, bilirubin and albumin levels rely on nutritional status, drug use, or cholestasis and might not be considered reliable markers of liver function (29).
In this study, there was also no correlation between miR-34a levels in the serum of all types of PFIC cases and complete blood count (CBC), prothrombin time test (PT), and international normalized ratio (INR). However, the results of the present study did not show any considerable link between serum miR-34a and GGT levels in the PFIC group, which is not in line with Rieger et al.’s results that observed a significant association between elevated GGT, age, cholestasis, and increased levels of miR-34a (27). This controversy between the results might be due to differences in the type of samples, gene expression analysis method, the type of liver disease, and severity of the disease.
Additionally, the results of the current study showed no relationship between miR-34a expression and receiving liver transplantation or other GI-related surgeries. Furthermore, miR-34a levels had no link with parents’ relation among PFIC cases.
To the best of our knowledge, this is the first study performed to evaluate the expression level of miR-34a in PFIC patients from different regions and ethnicities in Iran. However, there are some limitations in the current study. One of these limitations was the small sample size in both case and control groups. Since this study was cross-sectional, long-term follow-up could not be assessed. Due to the aforementioned limitations, further investigations with a large population of PFIC patients and healthy cases are recommended to extensively analyze the potential of miR-34a as a biomarker for PFIC diagnosis and prognosis.
In summary, the results of the present study demonstrated that the serum level of miR-34a increased significantly in patients with PFIC in comparison to that of healthy subjects. Therefore, miR-34a might be a potential non-invasive biomarker for PFIC diagnosis, treatment, and prognosis. miR-34a might also serve as a biomarker of fibrosis due to any cause, not essentially PFIC. Adding a third group with liver fibrosis due to causes other than PFIC would be very helpful.