1. Background
Leptospirosis is a significant zoonotic illness caused by Leptospira spirochetes, with over 300 pathogenic serovars identified worldwide. Humans typically contract the infection through contact with water or soil contaminated by the urine of mammals such as dogs, cattle, pigs, or rodents (1). Annually, it is estimated that over one million cases and nearly sixty thousand deaths occur globally (2). Infection commonly results in asymptomatic or self-limiting disease in both adults and children. However, severe cases can manifest as a fatal illness with or without pulmonary hemorrhage, typically characterized by jaundice and renal dysfunction (known as Weil's disease). The clinical course of the disease is biphasic; the acute or bacteremic phase lasts for about a week, followed by the immune phase characterized by antibody production and urinary excretion of leptospires. Most complications arise during the immune phase of the disease, attributed to the localization of leptospires in tissues (1-3). Diagnosis of leptospirosis is usually based on serological tests using ELISA (4, 5). Leptospires are generally not cultured from blood, cerebrospinal fluid, or urine using standard culture media, even with the use of specialized media. Results from culture are available with low sensitivity and only after several weeks. Conversely, molecular techniques, such as polymerase chain reaction (PCR) exhibit high sensitivity and specificity, particularly in the early stages (5).
Worldwide, approximately 500,000 cases of severe leptospirosis are reported annually, but the true incidence of infection is believed to be higher due to diagnostic challenges and underreporting. Although most cases of leptospirosis are mild and self-limiting, the disease is estimated to cause 60,000 deaths globally each year (2). There is growing concern that leptospirosis outbreaks may become more frequent due to climate change (6, 7).
Despite published case series in Turkey, the actual incidence of leptospirosis remains unknown (8-10). While the annual incidence of the disease in temperate regions of the world ranges from 0.1 to 1 per 100,000 population, it can reach 10 to 100 per 100,000 in tropical regions with heavy rainfall (11). Turkey falls within the temperate climate zone, with an estimated incidence of 0.1 to 1 per 100,000 population (8). Although the true incidence is uncertain, numerous cases and case series have been reported from various regions in Turkey, including the Marmara, Black Sea, Eastern Anatolia, and Çukurova regions (12).
The Southeastern region of Turkey is characterized by fertile soils and relies heavily on agriculture and animal husbandry as primary sources of livelihood. The region experiences a mild to hot climate. The higher proportion of rural population in this area may contribute to the elevated incidence of leptospirosis.
2. Objectives
Between January 1, 2020, and December 31, 2021, the medical records of all patients under 18 years of age diagnosed with leptospirosis were retrospectively reviewed. This study aimed to investigate the demographic, clinical, and laboratory findings of pediatric patients diagnosed with leptospirosis in the Southeastern region of Turkey.
3. Methods
The medical records of all patients under 18 years old diagnosed with leptospirosis (according to the International Statistical Classification of Diseases and Related Health Problems, ICD-10) between January 1, 2020, and December 31, 2021, were retrospectively reviewed. The clinical findings, risk factors, laboratory findings, treatments, and treatment outcomes of the patients were recorded. The patients' risk factors (contact with fresh water up to three weeks before diagnosis, occupational exposure) were documented. None of the patients had a history of travel. All of them were found to be working at wells in cornfields, assisting their families with irrigation work, or being involved in well-related activities.
3.1. Inclusion Criteria
- Patients admitted between January 1, 2020, and December 31, 2021
- Patients under 18 years old
- Patients diagnosed with leptospirosis based on PCR results
3.2. Exclusion Criteria
- Patients older than 18 years old
- Patients suspected of having leptospirosis but without PCR results
- Patients with co-morbidities
- Patients with missing data
Molecular methods such as PCR, which detect bacterial DNA in leptospirosis and have higher sensitivity and specificity rates compared to other diagnostic methods, have been increasingly accepted in recent years. Due to the advantages of these methods, their use in diagnosing leptospirosis is increasing (13).
Urine and blood samples were collected from all patients exhibiting clinical findings compatible with leptospirosis and were sent to the health directorate for microscopic agglutination testing and PCR. Patients with positive PCR results were included in the study. The results of examinations, treatments administered, and the inpatient or outpatient follow-up of our patients were documented. The choice of antibiotic for patients was based on the clinical experience and differential diagnosis of the attending physician, and no interventions were made due to the retrospective nature of the study. Since blood and urine samples from suspected leptospirosis patients in our hospital are sent to the health directorate, there is a delay in obtaining results for clinical follow-up. Therefore, empirical antibiotic therapy was continued until the results were available.
Ethics committee approval for the study was obtained from the Mardin Artuklu University Non-Interventional Clinical Research Committee on September 14, 2022, with the reference number 66601.
3.3. Statistical Analysis
The data obtained were analyzed using the SPSS 25.0 statistical package program. Descriptive statistical methods such as percentages, standard deviation, frequency, mean, minimum, and maximum values were used to evaluate the data. Additionally, the Kolmogorov-Smirnov test was conducted to assess the normal distribution of the data. The analysis revealed that the data exhibited a normal distribution.
4. Results
In this two-year study, 36 confirmed cases of leptospirosis in individuals under 18 years of age were identified in an area with approximately half a million inhabitants in southeastern Turkey. The mean age of the patients was 15 years, and they were all male. None of the patients had a history of travel, and all were found to be working at wells in cornfields, assisting with irrigation work, or involved in related activities. Of the patients, 14 (39%) were Syrian nationals, and 22 (61%) were Turkish citizens. While 3 (8%) of the patients lived in urban areas, 33 (92%) lived in rural areas. None of the patients required intensive care, with 12 (33%) receiving outpatient clinic follow-up and 24 (67%) being hospitalized and monitored (Table 1). All patients showed improvement with treatment, and there were no instances of intensive care or mortality. No additional issues were identified during outpatient clinic follow-up visits.
| Variables | Values a |
|---|---|
| Characteristics | |
| Age, y | 15 (10.5 - 17.5) |
| Gender | |
| Male | 36 (100) |
| Exposure | |
| Occupational (well-related) | 36 (100) |
| Area of living | |
| Urban | 3 (8) |
| Rural | 33 (92) |
| Hospitalization | |
| Intensive care | 0 |
| Medical ward | 24 (67) |
| Outpatient | 12 (33) |
a Values are expressed as mean (min – max) or No. (%).
Leptospirosis is more prevalent during the summer and early fall. Our patient group consisted of children working in irrigation, and all cases occurred during the summer.
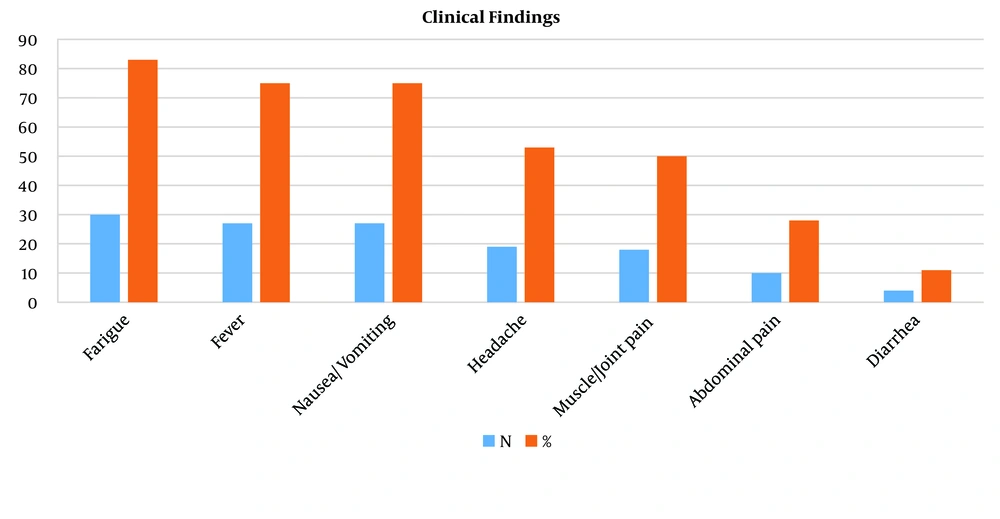
Upon reviewing the clinical findings of the patients, the most common complaints were fatigue (83%), fever (75%), and nausea/vomiting (75%). Additionally, patients reported symptoms such as headache (53%), muscle/joint pain (50%), abdominal pain (28%), and diarrhea (11%) (see Figure 1).
In terms of laboratory tests, all patients tested positive for C-reactive protein (CRP), with a high rate (61%) exhibiting significantly elevated levels (CRP > 100). The second most common laboratory abnormality was lymphopenia, detected in 31 (86%) patients. Furthermore, thrombocytopenia was observed in the majority of patients (64%). Additional laboratory findings included elevated levels of aspartate aminotransferase (AST) in 47% of patients, hyperbilirubinemia in 44%, leukocytosis in 39%, elevated alanine aminotransferase (ALT) in 28%, leukopenia and anemia in 11%, and elevated creatinine in 8% (Table 2).
| Test | No. (%) | Mean (SD) |
|---|---|---|
| Hemoglobin < 12.4 g/dL | 4 (11) | 13.9 (1.37) |
| Lokositosis > 1 0500 | 14 (39) | 9730 (3977) |
| Lokopenia < 4 500 | 4 (11) | 9730 (3977) |
| Lymphopenia < 1 100 | 31 (86) | 856 (368) |
| Trombocytopenia < 150.000 | 23 (64) | 146.000 (67000) |
| Trombocytopenia < 50.000 | 2 (6) | 146.000 (67000) |
| Hyponatremia < 132 | 6 (17) | 135 (2.71) |
| Hyperbilirubinemia > 1.2 | 16 (44) | 1.5 (1.44) |
| Hyperbilirubinemia > 3 | 2 (6) | 1.5 (1.44) |
| AST > 45 | 17 (47) | 58 (46.4) |
| AST > 100 | 4 (11) | 58 (46.4) |
| ALT > 45 | 10 (28) | 44 (44.9) |
| ALT > 100 | 3 (8) | 44 (44.9) |
| Creatinine > 1.2 | 3 (8) | 1 (0.18) |
| CRP > 100 | 22 (61) | 131 (89) |
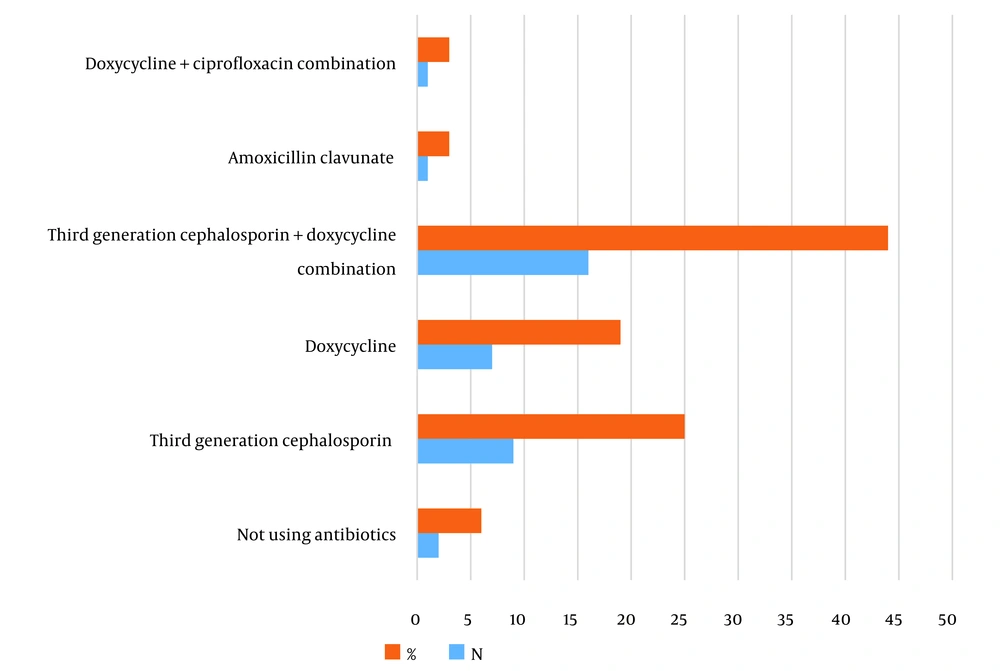
Thirty-four (96%) of the patients received antibiotic therapy for leptospirosis. The most commonly prescribed antibiotics were third-generation cephalosporins (ceftriaxone or cefotaxime) in combination with doxycycline, which was used in 16 patients (44%). Third-generation cephalosporins alone were administered to 9 patients (25%). Sole administration of doxycycline was prescribed to 7 patients (19%), while one patient (3%) received amoxicillin-clavunate, and another patient (3%) received a combination of doxycycline and ciprofloxacin. The treatment regimens for 2 patients could not be determined (Figure 2).
5. Discussion
In an area with approximately half a million inhabitants in southeastern Turkey, 36 confirmed cases of leptospirosis among individuals under the age of 18 were identified. The number of cases detected in our study exceeds the rate of 0.14 per 100,000 population reported in the annual epidemiology report for leptospirosis published by the European Center for Disease Prevention and Control in 2020, and it is higher than the estimated range of 0.1 - 1 for temperate regions (11-14). While there is no available data for Turkey as a whole to make a comparison, the higher proportion of rural population in the southeast region may contribute to the increased incidence. Additionally, climate change may also play a role in the rise of rare diseases such as leptospirosis (6, 7). Individuals at risk include farmers, those in contact with livestock, individuals in close proximity to rodents, and people residing in areas with inadequate sanitation (2).
Special precautions should be taken to prevent transmission of the disease from infected animals to humans, particularly focusing on rodents, which are the primary hosts of the disease. Occupational, recreational, and sports activities should involve the use of protective equipment such as boots, gloves, and goggles. Extra care should be taken in cases where there is a compromise in skin integrity. It is presumed that adherence to these precautions may be lower in the pediatric age group.
Contrary to findings in studies conducted in Germany by Brehm et al. and Greece by Gkentzi et al. where a significant portion of cases were travel-related (79% and 11% respectively), all cases in our study were attributed to occupational risks, with no travel-related cases detected. This discrepancy may be due to the low frequency of travel in southeastern Turkey, influenced by social, cultural, and economic factors. All cases in our study were associated with exposure to irrigation wells on agricultural lands (15, 16).
In the study conducted by Klement-Frutos et al. investigating cases in New Caledonia from 2006 to 2016, the number of male cases was twice that of females. Similarly, in the study by Guerrier et al., 79% of pediatric cases were male (17, 18). In our study, all cases were male, which we attributed to contamination related to well and field irrigation, with boys often assisting their families with these tasks being predominant.
Generally, the majority of leptospirosis patients present to hospitals with acute fever syndrome of unknown origin. In the study by Gkentzi et al., 93% of cases presented with fever (16). Similarly, in our study, as documented in the literature, the most common complaints among patients were fatigue and fever. While leptospirosis can lead to severe conditions like Icteric leptospirosis or Weil's Syndrome characterized by multiorgan damage, jaundice, renal failure, cardiac involvement, and pulmonary hemorrhage, these were not observed in our study. Jaundice was detected in 16 (44%) patients, but no cases of Weil's syndrome were identified. Consistent with findings in the literature, the disease severity in our patients was milder compared to adults, and none of the patients required intensive care (18).
Leptospirosis typically results in neutropenia, lymphopenia, and thrombocytopenia within the first five days of illness, with significant increases observed in all counts thereafter. Anemia is also commonly observed (19). In the study by De Silva et al., thrombocytopenia was noted in 75% of patients, while in our study, consistent with previous findings, thrombocytopenia was prevalent among most cases. Although lymphopenia is considered atypical in leptospirosis, it was found in the majority of our patients (20). Notably, anemia was observed in only 11% of cases in our study, contrasting with the 66% reported in the study by Gkentzi et al. This discrepancy may be attributed to the pediatric population exclusively included in our study (16).
Consistent with the literature, our study revealed elevated CRP values in 61% of patients, akin to those observed in gram-negative pathogen-associated bacteremia (16). Regarding liver involvement, liver function tests showed elevations in approximately half of the patients, with values exceeding 100 U/L detected in 10% of cases. This is indicative of the typically milder course of leptospirosis in children (18).
In previous studies, antibiotic treatment for leptospirosis typically involved intravenous administration for 5 - 7 days, with ampicillin being administered to 43 patients and cefotaxime to 17 patients (18). In a study conducted in Greece, empirical antibiotic therapy was initiated in 40 patients diagnosed with leptospirosis. Among these, 14 patients received ceftriaxone, 9 patients were treated with doxycycline, 5 patients received a combination of ceftriaxone and doxycycline, and 12 patients were given other antibiotics such as meropenem, quinolone, or macrolide. Treatment was not administered to 5 patients (16). In our study, 16 patients received a combination of third-generation cephalosporin (ceftriaxone or cefotaxime) and doxycycline, 9 patients were treated solely with third-generation cephalosporin (ceftriaxone or cefotaxime), and 7 patients received doxycycline alone. Additionally, one patient was treated with amoxicillin-clavulanic acid, and another received a combination of doxycycline and ciprofloxacin.
Leptospirosis exhibits a higher prevalence in the summer and early fall months (6). Our patient cohort comprised children engaged in irrigation work, with all cases occurring during the summer.
5.1. Conclusions
Our study represents the first investigation into the epidemiology, clinical manifestations, laboratory findings, and treatment of leptospirosis in southeast Turkey. Given the retrospective nature of the study, caution is warranted in interpreting the results. Our study included symptomatic patients who tested positive for PCR, and considering the typically milder clinical presentation of leptospirosis in children, the actual incidence of the disease may be higher than reported. Although data on the incidence of leptospirosis in Turkey are limited, it is worth noting that the incidence in the southeast region may be higher due to intensive use of well water, as observed in our study.
The clinical presentation of leptospirosis varies widely, and symptoms and findings may be milder and less certain in children. Therefore, clinicians should consider leptospirosis when evaluating patients, particularly those with occupational exposures, and should not hesitate to request testing.
Leptospirosis poses a significant public health concern. Precautions should be taken to prevent transmission of the disease from carrier animals, particularly rodents, to humans. This includes the use of protective equipment such as boots, gloves, and goggles during occupational, recreational, and sporting activities.
5.2. Limitations of the Study
This study is limited by its retrospective design and reliance on data from a single center. Additionally, as it was conducted in a hospital in the southeastern region, the findings may not be generalizable to the entire population. Furthermore, there is a paucity of research on leptospirosis in pediatric patients. Future epidemiological studies with more comprehensive data are warranted to further elucidate this topic.