1. Background
Dystrophin-related muscular dystrophies are a group of diseases caused by mutations in the dystrophin gene that are inherited in an X-linked recessive manner. Dystrophin is produced mainly in skeletal and cardiac muscles and is responsible for protecting muscle fibers (1). Dystrophin and other muscle membrane proteins form a dystrophin-associated protein complex that stabilizes the muscle cell membrane. In the absence or reduction of dystrophin, the muscle cell membrane becomes more fragile and prone to tearing and disintegration during muscle fiber contraction (2). Historically, dystrophinopathies have been classified into two main phenotypic groups: Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD)(3).
The incidence of DMD is 1 in 3,500 to 5,000 live male births, making it the most common muscular dystrophy of childhood (4). Duchenne muscular dystrophy is a severe form of dystrophin-related muscular dystrophy that begins in early childhood. An affected child is usually diagnosed before the age of 4, with early symptoms including delayed ability to sit, difficulty standing independently, and difficulty learning to speak (1). Continued muscle damage leads to progressive muscle weakness, resulting in loss of ambulation (LoA), wheelchair dependence by age 13, and death before the third decade (5). The most common causes of death in DMD are respiratory complications and heart failure resulting from progressive cardiomyopathy (1).
Becker muscular dystrophy typically has a milder and more variable phenotype. The deleterious effect of disease-causing mutations in the dystrophin gene is less severe than in DMD because dystrophin can be produced in smaller amounts or with reduced function. In BMD, skeletal muscle weakness progresses gradually and usually begins around age 8. The most common cause of death in BMD is heart failure (1).
Muscle biopsy was previously the primary diagnostic tool for DMD/BMD. However, it has been replaced by molecular tests that identify the type of mutation in the dystrophin gene (5). The dystrophin gene consists of 79 exons located in the p21 region of the X chromosome. Approximately 65% of mutations in the dystrophin gene result from deletions of one or more exons. Exon duplications constitute 6% of mutations, while nonsense mutations account for approximately 13% (6).
Dystrophin maintains the structural stability of the muscle, and its absence causes the release of creatine kinase (CK) into the circulation, triggering muscle rupture and severe progressive muscle degeneration. As a result, CK, a biomarker of muscle damage, increases in the serum and is often the first indicator leading to the diagnosis of DMD and BMD. Creatine kinase is an essential and permanent indicator of muscle breakdown and may not be related to the level of motor function (7). In DMD patients, CK is often elevated 50 to 100 times the normal level. Serum CK may sometimes be elevated without clinical symptoms or weakness. Since patients with BMD can perform much more motor activity, CK elevations may be much higher than in patients with DMD (6). Additionally, muscle breakdown causes transaminases such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) to be released into the circulation.
Although ALT and AST are markers of hepatocellular damage, they are also highly expressed in muscle cells. Therefore, elevated serum transaminase levels in DMD patients are well documented and may indicate the destruction of the muscle membrane. For this reason, muscle disease should be considered in children with elevated transaminase levels (7). There is no definitive treatment for DMD yet, but many experimental treatment strategies are under investigation. Current DMD treatment focuses on a multidisciplinary approach to managing symptoms and improving quality of life and function (8). The only drugs that have been shown to definitively improve muscle strength and function in DMD patients are glucocorticoids, with evidence-based studies confirming their effectiveness.
Although no cure exists for DMD, its monogenic nature and well-documented history have made it a prime target for genetic and other biological treatments. This has led to numerous therapeutic interventions from various modalities. A wide variety of biological and genetic treatments are currently being investigated for DMD, including exon skipping therapy, microdystrophin production, stop codon readthrough therapy, CRISPR-based gene editing, cell-based therapy, and utrophin editing (9).
2. Objectives
The diagnosis of dystrophinopathy is based on clinical, laboratory, biopsy, and genetic testing. Although elevated transaminase levels can aid in diagnosis, they can sometimes lead to diagnostic confusion and unnecessary further tests. In this study, we aim to share our clinical experiences by evaluating the clinical and laboratory features, diagnostic methods, treatments, and prognostic features of our DMD/BMD patients, as well as the laboratory methods that can guide early diagnosis.
3. Methods
3.1. Study Type and Study Group
In this study, the medical records of 39 children diagnosed with DMD/BMD and followed up at Bursa Uludağ University Faculty of Medicine Child Neurology Clinic between August 2018 and September 2023 were evaluated retrospectively. This study was approved by the Ethics Committee of Bursa Uludağ University (2023-17/70).
3.2. Inclusion/Exclusion Criteria
All cases diagnosed with DMD/BMD in our clinic and with available data were included in the study. Healthy or symptomatic carrier girls and patients whose medical records were inaccessible were not included. DMD or BMD was diagnosed based on genetic testing, muscle biopsy, and clinical phenotype. The genetic diagnosis methods included multiplex ligation-dependent probe amplification (MLPA) and next-generation sequencing (NGS) for boys clinically suspected of DMD/BMD but with negative MLPA results.
3.3. Data Collection and Definitions
The medical records of the patients were collected retrospectively through the hospital information system, and missing clinical or laboratory data could not be evaluated. Data on the patients' clinical history, including age, gender, consanguinity, family history, age at symptom onset, age at diagnosis, follow-up period, initial symptoms, and examination findings (muscle weakness, calf muscle hypertrophy, Gowers' sign), as well as LoA age, wheelchair dependence, electromyography (EMG) data, echocardiography (ECHO) data, muscle biopsy data, genetic testing data, serum CK, AST, and ALT levels, and steroid treatment used (prednisolone or deflazacort), ataluren or other treatment data, and accompanying comorbidities were collected.
3.4. Statistical Analysis
Data were analyzed using IBM-SPSS Statistics (version 25). The suitability of continuous variables for normal distribution was examined with the Shapiro-Wilk test. According to the results of the normality test, variables that comply with normal distribution are presented with their mean and standard deviation, while variables that do not comply with normal distribution are given with their median, minimum, and maximum values. For comparisons between groups, the Mann-Whitney U test and Kruskal-Wallis test were used based on the normality test results. Categorical variables were compared between groups using the Pearson chi-square test and the Fisher-Freeman-Halton test. Relationships between numerical variables were examined with correlation analysis, and the Spearman correlation coefficient was calculated. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Participants’ Demographics
In this study, we reviewed 39 patients with DMD and BMD, comprising 29 (74.4%) DMD patients and 10 (25.6%) BMD patients. All participants were male, with an age range of 9.88 years (2.4 - 19.4 years). The average age of DMD patients was 10.54 years, while that of BMD patients was 7.97 years. A family history was reported in 14 patients (35.9%): Eight in the DMD group and six in the BMD group. A history of motor delay was reported in 10 patients (25.6%), all in the DMD group. Table 1 summarizes the demographic and clinical data of the participants.
| Variables | All Patients (N = 39) | DMD (n = 29) | BMD (n = 10) |
|---|---|---|---|
| Age (y) | 9.88 ± 4.73 (2.4 - 19.4) | 10.54 ± 4.72 (2.4 - 19.4) | 7.97 ± 4.43 (3.1 - 16.6) |
| Consanguinity | 5 (12.8) | 5 (17.2) | 0 (0) |
| Family history for DMD/BMD | 14 (35.9) | 8 (27.6) | 6 (60) |
| Motor delay history | 10 (25.6%) | 10 (34.5) | 0 (0) |
| Age of symptom onset (y) | 3.44 ± 2.7 (1 - 12.2) | 3.01 ± 1.8 (1 - 8) | 5.56 ± 5.1 (1.5 - 12.2) |
| Age of diagnosis (y) | 5.35 ± 2.98 (0.8 - 12.6) | 4.91 ± 2.59 (0.8 - 9.8) | 6.49 ± 3.74 (1.6 - 12.6) |
| Corticosteroids treatment initiation (y) | 6.84 ± 2.31 (4 - 13) | 6.47 ± 1.93 (4 - 12.8) | 11.25 ± 2.47 (9.5 - 13) |
| Age at loss of ambulation (y) | 10.8 ± 1.31 (8.6 - 12.5) | 10.8 ± 1.31 (8.6 - 12.5) | _ |
| Follow-up period (y) | 4.62 ± 4.26 (0.08 - 12.1) | 5.72 ± 4.29 (0.08 - 12.1) | 1.43 ± 2.09 (0.08 - 7.16) |
| Symptoms and findings on admission | |||
| CK elevation (accompanied AST/ ALT elevation) | 12 (30.8) | 8 (27.6) | 4 (40) |
| Family history for DMD/BMD | 5 (12.8) | 4 (13.8) | 1 (10) |
| Difficulty walking | 17 (43.6) | 15 (51.8) | 2 (20) |
| Difficulty in climbing stairs | 14 (35.9) | 13 (44.8) | 1 (10) |
| Getting tired quickly | 11 (28.2) | 11 (37.9) | 0 |
| Frequent falls | 5 (12.8) | 5 (17.2) | 0 |
| Difficulty in running | 4 (10.3) | 4 (13.8) | 0 |
| Toe-walking | 2 (5.1) | 2 (6.9) | 0 |
| Inability to jump | 2 (5.1) | 2 (6.9) | 0 |
| Myalgia | 2 (5.1) | 0 | 2 (20) |
| Pseudohypertrophy | 30 (76.9) | 24 (82.8) | 6 (60) |
| Gowers' sign | 11 (28.2) | 11 (37.9) | 0 |
| Proximal muscle weakness | 8 (20.5) | 8 (27.6) | 0 |
a Values are expressed as No. (%) or mean ± SD.
4.2. Symptoms Onset
The age of symptom onset was 3.44 years (range: 1 - 12.2 years), with an average of 3.01 years in DMD patients and 5.56 years in BMD patients. In this study, 17 cases were initially asymptomatic (12 DMD patients and five BMD patients). Of these, 12 cases (eight DMD and four BMD) were referred to our clinic due to elevated CK levels (accompanied by AST/ALT elevation), and five cases (four DMD and one BMD) were referred due to a family history of DMD/BMD.
The main reasons for referral in symptomatic patients were muscle weakness observed by family members, including difficulty walking (17/22), difficulty climbing stairs (14/22), getting tired quickly (11/22), frequent falls (5/22), difficulty running (4/22), toe-walking (2/22), inability to jump (2/22), and myalgia (2/22). During the initial physical examination, pseudohypertrophy was detected in 30 cases (76.9%), Gowers' sign in 11 cases (28.2%), and proximal muscle weakness in eight cases (20.5%). Symptoms and findings present at the onset of the disease are reported in Table 1.
4.3. Laboratory Tests
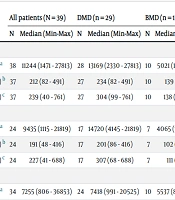
CK levels were elevated in all patients. Serum ALT and AST levels were also elevated in all patients. Serum enzyme levels at the initial visit, diagnosis, and last control are reported in Table 2. CK, AST, and ALT levels were found to be significantly higher in DMD patients compared to BMD patients at the initial visit and diagnosis. However, this relationship was not observed in the last control values (Table 2).
| Enzymes | All patients (N = 39) | DMD (n = 29) | BMD (n = 10) | P | |||
|---|---|---|---|---|---|---|---|
| N | Median (Min-Max) | N | Median (Min-Max) | N | Median (Min-Max) | ||
| Initial | |||||||
| CK (U/L) a | 38 | 11244 (1471 - 27813) | 28 | 13169 (2330 - 27813) | 10 | 5021 (1471 - 12994) | < 0.001 |
| AST (U/L) b | 37 | 212 (82 - 491) | 27 | 234 (82 - 491) | 10 | 139 (83 - 415) | 0.003 |
| ALT (U/L) c | 37 | 239 (40 - 761) | 27 | 304 (99 - 761) | 10 | 138 (40 - 426) | 0.002 |
| Diagnosis | |||||||
| CK (U/L) a | 24 | 9435 (1115 - 21819) | 17 | 14720 (4145 - 21819) | 7 | 4065 (1115 - 10429) | 0.003 |
| AST (U/L) b | 24 | 191 (48 - 416) | 17 | 201 (86 - 416) | 7 | 102 (48 - 362) | 0.028 |
| ALT (U/L) c | 24 | 227 (41 - 688) | 17 | 307 (68 - 688) | 7 | 111 (41 - 213) | 0.001 |
| Last control | |||||||
| CK (U/L) a | 34 | 7255 (806 - 36853) | 24 | 7418 (991 - 20525) | 10 | 5537 (806 - 36853) | 0.75 |
| AST (U/L) b | 35 | 146 (30 - 820) | 25 | 123 (30 - 328) | 10 | 180 (41 - 820) | 0.22 |
| ALT (U/L) c | 35 | 208 (34 - 509) | 25 | 183 (34 - 509) | 10 | 209 (40 - 352) | 0.9 |
a CK (U/L) = 12 - 200.
b AST (U/L) = 18 - 36.
c ALT (U/L) = 9 - 25.
A significant positive correlation was detected between the initial CK value and the initial AST and ALT values (r = 0.82, P < 0.001 and r = 0.83, P < 0.001, respectively). Similarly, a significant positive correlation was found between the diagnostic CK value and the diagnostic AST and ALT values (r = 0.87, P < 0.001 and r = 0.82, P < 0.001, respectively). Additionally, a significant positive correlation was observed between the last CK and the last AST and ALT values (r = 0.91, P < 0.001 and r = 0.86, P < 0.001, respectively).
In our cohort, 12 patients presented with high CK and transaminase levels; two of them were followed for approximately two years in pediatric gastroenterology due to elevated transaminase levels. A liver biopsy was performed on one patient with a preliminary diagnosis of chronic hepatitis, but the biopsy result was normal. Later, due to the patient's high CK level, it was suspected that he might have a muscle disease, and the patient was referred to the pediatric neurology clinic. Another patient with elevated transaminase levels first presented to us with seizure complaints at five months old and was diagnosed with epilepsy. It was initially thought that the high transaminase levels were related to the antiepileptic drugs used. His treatment was discontinued, but his transaminase elevation did not improve. CK elevation was detected six months after the first visit, leading to a suspicion of muscle disease.
EMG was performed on 12 patients; 9 (75%) had myopathic findings, while three patients (25%) had normal EMG findings. ECHO was performed on 34 patients; 5 (14.7%) had abnormal ECHO findings (1 dilated cardiomyopathy, 2 left ventricular systolic dysfunction, 1 left ventricular hypertrophy, and 1 minimal aortic insufficiency). Respiratory function tests were performed on 14 patients; 11 (78.6%) had normal results, and three did not cooperate.
4.4. Diagnosis
The definitive diagnosis of the cases was made by muscle biopsy, which revealed dystrophic changes and a deficiency or absence of dystrophin in 6 cases, and by genetic tests in 33 cases. In 5 of the 6 cases diagnosed by muscle biopsy, the diagnosis was confirmed by genetic tests. The age of diagnosis was 5.35 years (range: 0.8 - 12.6 years), with an average of 4.91 years in DMD patients and 6.49 years in BMD patients. The follow-up period was 4.62 years (range: 0.08 - 12.1 years), with an average of 5.72 years in DMD patients and 1.43 years in BMD patients.
4.5. Genetic Characteristics
The diagnosis method was multiplex ligation-dependent probe amplification (MLPA) in 32 cases (84.2%), while next-generation sequencing (NGS) was used for boys who were clinically suspected of DMD/BMD but had negative MLPA results. NGS was the diagnostic tool in 6 cases (15.8%). Regarding the type of mutation, deletion mutations were found in 30 cases (78.9%), duplications in 2 cases (5.3%), nonsense mutations in 5 cases (13.2%), and a frameshift mutation in 1 case (2.6%). The most common single exon deletion was exon 45 (3 cases), and the most common multiexon deletions were exons 45 - 48 (4 cases). Table 3 summarizes the genetic characteristics.
| Genetic Mutation Types (del) | No. (%) |
|---|---|
| 2 dup | 1 (2.6) |
| 3 - 8 | 1 (2.6) |
| 8 - 30 dup | 1 (2.6) |
| 10 - 20 | 2 (5.3) |
| 18 - 34 | 1 (2.6) |
| 34 | 1 (2.6) |
| 45 | 3 (7.9) |
| 45 - 48 | 4 (10.5) |
| 45 - 50 | 1 (2.6) |
| 45 - 52 | 1 (2.6) |
| 45 - 55 | 1 (2.6) |
| 46 - 49 | 2 (5.3) |
| 46 - 51 | 1 (2.6) |
| 46 - 55 | 1 (2.6) |
| 48 - 49 | 2 (5.3) |
| 48 - 50 | 3 (7.9) |
| 45 - 52 | 1 (2.6) |
| 49 - 52 | 1 (2.6) |
| 49 - 54 | 1 (2.6) |
| 50 - 52 | 1 (2.6) |
| 51 - 55 | 1 (2.6) |
| 52 | 1 (2.6) |
| Nonsense | 5 (13.2) |
| Frameshift | 1 (2.6) |
4.6. Treatments
In this study group, 26 patients (66.7%) received corticosteroid treatment (24 patients with DMD and 2 patients with BMD). Specifically, 24 patients received prednisolone and 2 patients received deflazacort. Seven patients (17.9%) (2 patients with DMD and 5 patients with BMD) had never received corticosteroid treatment, and six patients (15.4%) (3 patients with DMD and 3 patients with BMD) were too young for such treatment. The mean age at the start of corticosteroid treatment was 6.84 ± 2.31 years (6.47 years in DMD and 11.25 years in BMD).
Regarding other treatments, 3 cases were currently receiving ataluren, 4 cases were receiving enalapril, 4 cases were receiving idebenone, 2 cases were receiving tamoxifen, 1 case was receiving digoxin, 1 case was receiving lisinopril, and 1 case was receiving carvedilol. Ataluren treatment was discontinued in 2 cases because they were non-ambulatory.
4.7. Prognosis
In this study, 13 patients (33.3%), all with DMD, were wheelchair-dependent, with a mean age of loss of ambulation (LoA) of 10.8 years (range: 8.6 - 12.5 years). Additionally, 5 patients had scoliosis, and 3 patients had epilepsy.
5. Discussion
This study retrospectively analyzed the demographics, clinical features, diagnostic methods, and genetic profiles of 39 DMD/BMD patients. Boys with DMD typically come to the clinician's attention between the ages of 2 and 5. While delayed walking is sometimes described, changes in gait are the most common symptom, with toe walking often prompting consultation with physiotherapists or orthopedic doctors before DMD is recognized (10). However, recent studies show that motor functions are impaired in DMD starting from infancy (11), and it is recommended to evaluate serum CK as part of routine screening in all infants with motor delay (12). The average age at diagnosis of DMD worldwide is approximately 4 - 5 years (13). Although detecting the onset of the disease is an essential part of the natural history of DMD, it is difficult to precisely measure the age of symptom onset because the delay in motor development is insidious. Symptoms progress slowly in the first few years of life.
In this study, most DMD/BMD patients reported a disease onset at an average age of 3.44 years (3.01 years in DMD, 5.56 years in BMD). In contrast, the age at confirmed diagnosis based on genetic testing or muscle biopsy was 5.35 years (4.91 years in DMD, 6.49 years in BMD). Similar to the literature, a diagnostic delay of approximately two years was detected in our DMD/BMD patients (5, 14). Family history was reported in 35.9% of patients (27.6% in DMD, 60% in BMD) and motor delay in 25.6% of patients (34.5% in DMD, 0% in BMD). Gan et al. reported a family history for BMD/DMD in 16.6% of their patients (15), while Rao et al. reported a family history in 22.73% of cases (16). Zamani et al. reported motor delay in 47.7% of cases (5), and Wonkam-Tingang et al. reported motor delay in 35.3% of cases (2). Therefore, all cases with a family history and motor delay should be evaluated for dystrophinopathies.
Creatine kinase levels were elevated in all patients, with AST and ALT levels also proportionally increased, consistent with CK elevation. The concentration of these enzymes in the serum of patients with DMD/BMD decreases with age, indicating progressive loss of muscle mass and disease progression. Therefore, in the early stages of DMD/BMD, CK and transaminases are elevated even before symptoms of muscle weakness appear. AST and ALT were strongly correlated with CK, suggesting that these enzymes may be valuable biomarkers for DMD/BMD diagnosis. At onset and diagnosis, CK, AST, and ALT levels in DMD patients were significantly higher than in BMD patients. However, no such relationship was found in the final control values. These findings are consistent with previous reports (7, 17, 18).
Persistent elevation of serum transaminase levels is well documented in various muscle diseases, including muscular dystrophies, inflammatory, and metabolic myopathies. Failure to consider muscle as the cause of high serum transaminase levels in these patients can lead to expensive and invasive hepatic procedures (such as liver biopsy), delayed recognition of occult or minimally symptomatic muscle disease, and unnecessary discontinuation of drug treatments (e.g., antibiotics, anticonvulsants) (18). Therefore, before conducting further examinations for liver diseases in cases with elevated transaminase levels, muscle diseases should be considered in the differential diagnosis. Evaluating cases for family history and CK elevation can protect patients from unnecessary, costly, invasive examinations and prevent delayed diagnosis. Muscle diseases should be included in the differential diagnosis algorithm for transaminase elevation.
Approximately 65% of dystrophin gene mutations are exon deletions, 6% are exon duplications, and 13% are nonsense mutations (6). This study detected 78.9% exon deletions, 5.3% exon duplications, 13.2% nonsense mutations, and 2.6% frameshift mutations. The most common single exon deletion was exon 45 (3 cases), and the most common multiple exon deletions were exons 45 – 48 (4 cases). The distribution and frequency of mutations in this study were similar to the results of other studies (2, 5, 19, 20).
In this study, 26 patients (24 with DMD and 2 with BMD) received corticosteroid treatment (24 with prednisolone and 2 with deflazacort), while seven patients had never received corticosteroid treatment, and six patients were too young for such treatment. The mean age at the start of corticosteroid treatment was 6.84 years (6.47 years for DMD and 11.25 years for BMD). Currently, corticosteroids are the only medications that improve muscle strength and function in DMD patients. Studies have confirmed their effectiveness, and evidence shows that corticosteroid use has increased in recent years (4, 21). The effectiveness of corticosteroid treatments, which can improve ambulation and cardiopulmonary function in DMD patients, has been confirmed by several studies. The age at which patients start corticosteroid treatment generally varies between 4 and 5 years (22).
The main treatment for DMD patients is glucocorticoids, which target the glucocorticoid receptor to produce anti-inflammatory effects by suppressing the NF-κB signaling pathway. Glucocorticoids have varying effectiveness and notable side effects such as weight gain, osteoporosis, cataracts, hypertension, and slowed bone growth. An innovative steroid, vamorolone, is currently being investigated as a potential alternative to corticosteroids, aiming to maintain the efficacy profile of corticosteroids while reducing their side effects. Ataluren is approved in many countries for the treatment of DMD. Ataluren is a disease-modifying molecule for stopping codon readthrough therapy and may help up to 10 - 15% of DMD patients carrying nonsense mutations as well as patients carrying frameshift mutations.
Eteplirsen, an antisense oligonucleotide drug that skips exon 51 from the dystrophin gene, was introduced after FDA approval in 2017, though there are reservations about its effectiveness. Other FDA-approved exon skipping drugs include ExonDys-51 for exon 51, Vyondys-53 and Viltolarsen for exon 53, and Amondys-45 for exon 45 skipping. However, no pharmacological drug can compensate for the lack of dystrophin in muscle fibers (23). Although no definitive cure is currently available for DMD, its monogenic nature and well-documented history have made it a prime target for genetic and other biological treatments, leading to numerous therapeutic interventions across various modalities (9).
For this reason, the genetic results of patients can be particularly useful in guiding them toward targeted treatments.
In this study, 13 patients (33.3%) were dependent on a wheelchair, with a mean age of loss of ambulation (LoA) of 10.8 years (range: 8.6 - 12.5 years). This finding is consistent with previous reports (2, 5, 19).
5.1. Limitations and Implications
The most significant limitations of this study were its retrospective design, the limited number of patients, and the fact that it was based on data from a single center. These factors impose limitations on the interpretation of the results.
A notable proportion (35.9%) of our patients have a family history of muscle disease. Given the recessive X-linked mode of inheritance, detailed questioning of family history is essential for diagnosing DMD/BMD patients. In our study, 12.8% of cases had no symptoms and sought medical advice solely due to a family history of muscle disease.
Another significant finding of our study is the necessity of including muscle diseases in the differential diagnosis of cases with elevated transaminase levels and investigating whether CK elevation accompanies them. In our cohort, 30.7% of cases presented with elevated CK and/or AST and ALT without any symptoms.
Although it is known that AST/ALT elevation occurs in muscle diseases, failing to include muscle diseases in the primary differential diagnosis of cases with AST/ALT elevation may lead to unnecessary and invasive examinations and a delay in diagnosis. Therefore, it is crucial to evaluate all patients with elevated AST/ALT for muscle diseases before conducting further examinations.
Genetic studies are needed to confirm the diagnosis in cases where dystrophinopathy is suspected. Muscle biopsy may be considered in a limited number of patients with a high likelihood of dystrophinopathy but no detectable pathology in genetic testing.
5.2. Conclusions
There is no cure for dystrophinopathies, but many experimental therapeutic approaches are currently in clinical trials. The treatment of DMD focuses on a multidisciplinary approach to managing symptoms and improving quality of life and function. Early recognition that increased serum transaminase and CK levels reflect muscle disease accelerates the diagnosis of underlying conditions and protects patients from unnecessary, invasive, and costly diagnostic testing.