1. Background
Neonatal intensive care units (NICU) are crucial for enhancing the survival chances of newborn babies by providing timely interventions for emerging problems. One of the most complex challenges in these settings is the management of endocrine disorders, which significantly contribute to morbidity and mortality in newborns (1). These disorders include issues with glucose homeostasis, thyroid function, and calcium regulation. The neonatal period, marked by the physiological shift from intrauterine to extrauterine life, adds complexity to these conditions, particularly in preterm and low birth-weight infants (2). These complications are often associated with preterm birth, low birth weight, very low birth weight (VLBW), and being small for gestational age (SGA) (3, 4).
Recent advancements in neonatal care techniques and diagnostic methods, particularly in genetic and biochemical analysis, have played a critical role in detecting and managing endocrine diseases. These advancements could significantly alter the patterns and prevalence of endocrine diseases in NICU settings over time. Despite these improvements, a comprehensive and updated profile of endocrine disorders in NICU populations is still lacking. The existing literature often limits endocrine disorders to isolated case studies or specific disease conditions, and there is a scarcity of comprehensive data on endocrine dysregulation in this vulnerable population (5, 6).
2. Objectives
This study aims to review and analyze the prevalence and patterns of a wide range of endocrine disorders in a NICU cohort to provide a detailed understanding of the recent epidemiology of these disorders and to establish a solid foundation for future research. Such research will guide clinical practice and health policies based on current knowledge. In this context, our study seeks to present the current status of endocrine diseases in the field of neonatal endocrinology.
3. Methods
This study is a retrospective analysis of hospitalization records from patients admitted to the NICU at Çanakkale Onsekiz Mart University Health Practice and Research Hospital, located in a city in the west of Türkiye. This city has a population of approximately 550,000, with the pediatric population constituting 18.7% of the total (7). The study included patients hospitalized for at least one day between January 1, 2016, and March 31, 2021, who were assigned an international classification of diseases (ICD) code for endocrinological problems. Patients who stayed in the hospital for less than 24 hours or whose records were incomplete in the electronic archive were excluded from the study.
The study established definitive diagnostic criteria to identify and categorize various endocrine disorders in neonates. Hypoglycemia was defined as a serum glucose level below 50 mg/dL in at least two consecutive measurements requiring treatment. Congenital hypothyroidism was diagnosed in patients with a thyroid-stimulating hormone (TSH) level exceeding 20 mIU/L on or after the fifth postnatal day. Hypothyroxinemia of prematurity was identified in premature infants who exhibited normal or low TSH levels along with low free thyroxine (FT4) levels below 0.9 ng/dL. Neonatal hyperthyrotropinemia (HTT) was characterized by TSH values ranging from 6 to 20 mIU/L and normal FT4 levels between 0.9 and 2.2 ng/dL, measured on or after the twenty-first postnatal day. Hypercalcemia was defined as a serum calcium level exceeding 11 mg/dL or ionized calcium above 1.35 mmol/L.
3.1. Statistical Analysis
Data collected from the medical records included gender, gestational week, birth weight, mode of delivery, postnatal age at hospitalization, duration of hospitalization, diagnoses made during hospitalization, and laboratory values. Descriptive statistics were utilized to summarize the data, with means and medians presented for continuous variables to depict central tendencies, and counts and percentages for categorical variables to reflect proportions within the cohort. To further quantify the precision and reliability of our findings, we calculated 95% confidence intervals (CIs) for key statistics.
4. Results
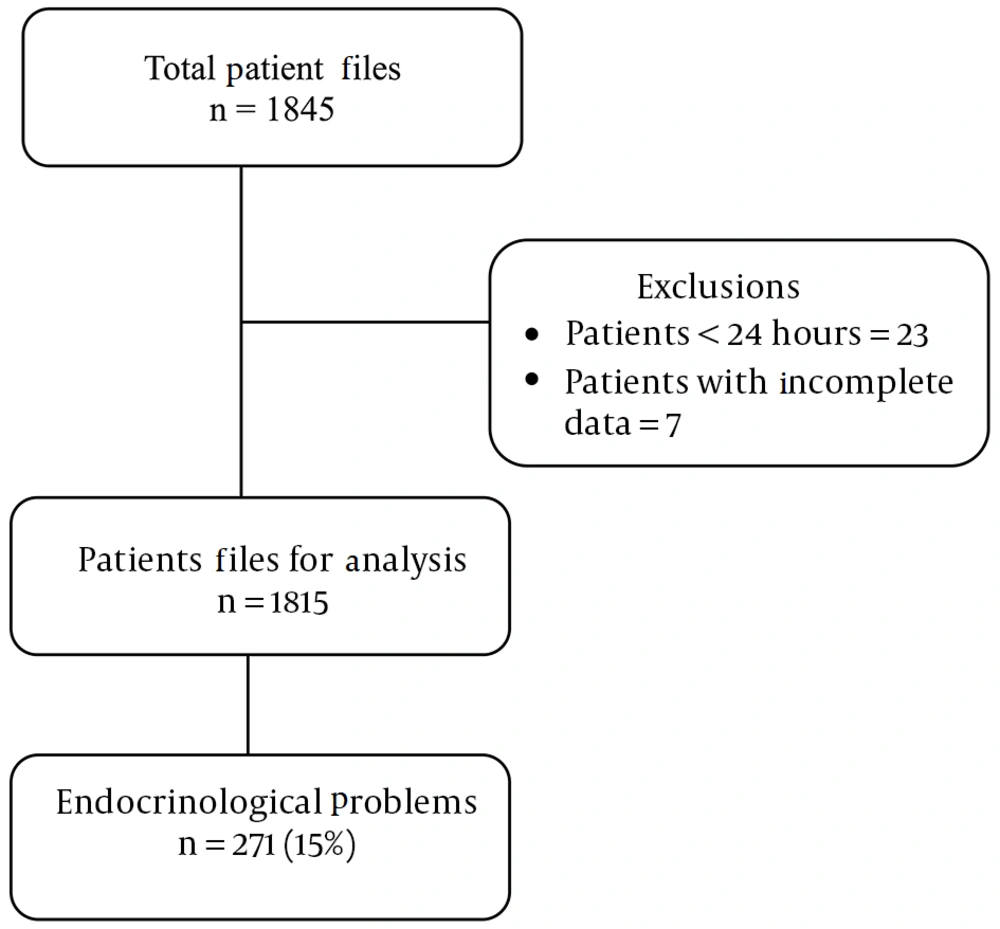
In this descriptive study, a total of 1,845 patient files from a five-year period were included. This dataset excluded 23 patients who stayed in the hospital for less than 24 hours and seven with incomplete electronic archival records. Endocrinologic problems were identified in 271 of the patient files reviewed (14.6%, CI: 13.15 - 16.38) (Figure 1). Of these patients, 127 were boys and 144 were girls, yielding a girl/boy ratio of 1.1. The mean birth weight of these cases was 2419 grams, with a range from 615 grams to 5980 grams. Of the cases, 214 were delivered by cesarean section (C/S), and 57 were delivered by normal spontaneous vaginal (NSV) delivery. Premature cases accounted for 53% of all cases. The median duration of hospitalization for neonates in the study was 13 days, with an interquartile range (IQR) from 8 to 32 days, as detailed in Table 1.
| Cohort Characteristics (n = 271) | Values |
|---|---|
| Gender | |
| Male | 127 (46.8) |
| Female | 144 (53.2) |
| Mean birth weight, g | 2419 |
| Birth weight range | 615 - 5980 |
| Delivery method | |
| Cesarean section | 214 (79) |
| Vaginal delivery | 57 (21) |
| Premature cases | 144 (53) |
| Hospitalization duration, day | 13 (8 - 32) |
a Values are expressed as No. (%) or median (1. quartile- 3. quartile).
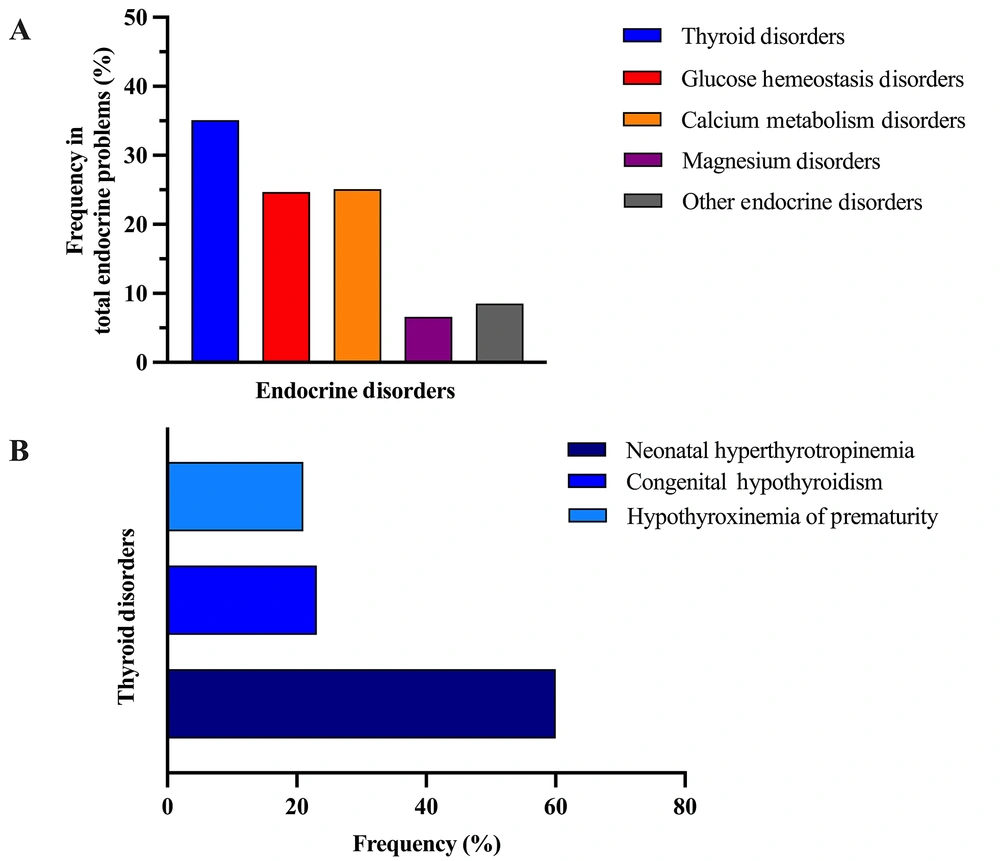
The most common endocrinologic problems observed in the study cohort were thyroid disorders (5.15%, CI: 4.23 - 6.25), calcium metabolism disorders (3.68%, CI: 2.92 - 4.64), glucose homeostasis disorders (3.63%, CI: 2.87 - 4.58), and magnesium disorders (0.98%, CI: 0.62 - 1.54) (Table 2). The types and frequencies of endocrine problems are presented separately for the entire group in Figure 2A.
| Endocrine Disorder | Total Number of Affected Babies | 95% CL |
|---|---|---|
| Thyroid disorders | 95 (5.15) | 4.23 - 6.25 |
| Neonatal hyperthyrotropinemia | 57 (3.09) | 2.30 - 3.88 |
| Congenital hypothyroidism | 22 (1.19) | 0.70 - 1.68 |
| Hypothyroxinemia of prematurity | 20 (1.08) | 0.61 - 1.56 |
| Glucose homeostasis disorders | 67 (3.63) | 2.87 - 4.58 |
| Calcium metabolism disorders | 68 (3.68) | 2.92 - 4.64 |
| Hypocalcemia | 55 (2.98) | 2.29 - 3.86 |
| Hypercalcemia | 13 (0.70) | 0.41 - 1.20 |
| Magnesium disorders | 18 (0.98) | 0.62 - 1.54 |
| Hypomagnesemia | 16 (0.87) | 0.54 - 1.40 |
| Hypermagnesemia | 2 (0.10) | 0.00 - 0.39 |
| Other endocrine disorders | 23 (1.25) | 0.83 - 1.86 |
| Phosphorus metabolism disorder | 14 (0.76) | 0.45 - 1.27 |
| Adrenal insufficiency | 5 (0.27) | 0.12 - 0.63 |
| Metabolic bone disease of prematurity | 4 (0.22) | 0.08 - 0.56 |
a Values are expressed as No. (%).
4.1. Glucose Homeostasis Disorders
In the NICU cohort, 67 patients were identified with disorders of glucose homeostasis, accounting for 24.7% (CI: 19.9 - 30.2) of all endocrinologic issues documented (Figure 2A). Hypoglycemia was prevalent, with 98.5% of this subgroup diagnosed with transient neonatal hypoglycemia. The underlying causes of hypoglycemia varied, including infants born to diabetic mothers (25.7%, CI: 16.7 - 37.3), sepsis (16.7%, CI: 9.6 - 27.4), prematurity (15.1%, CI: 8.4 - 25.2), SGA status (12.2%, CI: 6.3 - 22.1), large for gestational age (LGA) status (9.1%, CI: 4.2 - 18.4), transient hyperinsulinism (9.1%, CI: 4.2 - 18.4), nutritional deficiencies (6.1%, CI: 2.3 - 14.5), and polycythemia (6.1%, CI: 2.3 - 14.5). Notably, transient hyperinsulinism was confirmed in six cases of transient neonatal hypoglycemia, with three instances of refractory hypoglycemia necessitating additional treatment with diazoxide.
4.2. Calcium Metabolism Disorders
A total of 68 patients with calcium metabolism disorders were identified, comprising 25.1% (CI: 20.3 - 30.5) of those with endocrinologic issues (Figure 2A). The majority of these cases involved hypocalcemia (79.4%, CI: 68.3 - 87.3). Of the hypocalcemia cases, 49 (89%, CI: 80.1 - 95.9) were categorized as early neonatal hypocalcemia, and 6 (11%, CI: 5.2 - 22.2) as late neonatal hypocalcemia. Prematurity was the primary cause of early neonatal hypocalcemia (63.2%, CI: 49.2 - 75.3), with other contributing factors including sepsis, diabetic infancy, and asphyxia. All cases of late neonatal hypocalcemia were associated with magnesium disorders; four patients had hypomagnesemia, and two had hypermagnesemia.
Hypercalcemia was diagnosed in 14 patients, with half (50%) classified as idiopathic and the majority (42.8%) attributed to iatrogenic factors. The diagnosis of hypercalcemia typically occurred around postnatal day 12, with most cases presenting as mild hypercalcemia, characterized by calcium levels ranging from 11.1 - 13.6 mg/dL. Six cases were diagnosed based on ionized calcium values ranging from 1.46 - 1.60 mmol/L. In all instances, hypercalcemia was self-limiting, with symptoms including polyuria and constipation in three patients, and hypotonia in one patient.
4.3. Thyroid Disorders
Thyroid disorders (n = 95) accounted for 35.1% (CI: 29.6 - 40.9) of patients with endocrinological issues (Figure 2A). The most common thyroid disorder was neonatal HTT (Figure 2B), representing 3.09% (CI: 2.30 - 3.88) of all hospitalized cases and 57.9% (CI: 49.9 - 69.2) of the thyroid disorder group. Of these cases, 13 (13.6%) exhibited normal control TSH values before reaching postnatal day 21 (transient neonatal HTT), while in 22 cases, control TSH levels could not be assessed due to early discharge. In 20 cases (36.3%), elevated TSH levels persisted beyond postnatal day 21. However, FT4 values remained within the normal range throughout follow-up, categorizing these cases as neonatal HTT.
Cases of congenital hypothyroidism represented 1.19% (CI: 0.70 - 1.68) of all hospitalized cases and 23.1% (CI: 25.82 - 32.58) of the thyroid disorders group. The median age at diagnosis was 9.2 days, the median TSH value at diagnosis was 28.7 uIU/mL, ranging from 6.49 uIU/mL to over 100 uIU/mL, and the median FT4 value was 0.58 ng/dL.
4.4. Hypothyroxinemia of Prematurity
Hypothyroxinemia of prematurity accounted for 1.08% (0.61 - 1.56) of all inpatient cases and 21% (CI: 14.1 - 30.3) of the thyroid disorders group (Table 2) (Figure 2B). This condition developed in 3% of all hospitalized premature patients. The mean age at diagnosis was 5.4 days, the median gestational age was 29 weeks and 1 day (ranging from 24 to 36 weeks and 3 days), and the median birth weight was 1247.5 grams (ranging from 745 to 2290 g). The median FT4 level at diagnosis was 0.58 ng/dL (range 0.1 ng/dL to 0.8 ng/dL), and the median TSH level was 3.8 uIU/mL.
4.5. Magnesium Disorders
Magnesium metabolism disorders (n = 18) represented 6.6% (CI: 4.24 - 10.25) of patients with endocrinologic issues (Figure 2A). Among these, 16 had hypomagnesemia, and 2 had hypermagnesemia. The diagnosed cases of hypomagnesemia were predominantly caused by intrauterine growth restriction (IUGR) (n = 7, 43.7%), drug use (particularly aminoglycosides) in 7 cases (43.7%), and maternal diabetes in 2 cases (12.6%). Both cases of hypermagnesemia were attributed to parenteral nutrition, and the median serum magnesium value was 3.6 mg/dL.
4.6. Other Disorders
Additional endocrinologic disorders were identified in 23 cases, constituting 8.5% (CI: 5.72 - 12.41) of patients with endocrinologic issues (Figure 2A). Of these, 14 had phosphorus metabolism disorders, 5 had adrenal insufficiency (ACTH resistance syndrome: (1) congenital adrenal hyperplasia; and (2) central adrenal insufficiency in 2 cases), and 4 had premature metabolic bone disease.
5. Discussion
This descriptive study explored the range of endocrine problems in infants hospitalized in a NICU. The findings indicate that thyroid disorders, calcium disorders—with a notable prevalence of hypocalcemia—and glucose homeostasis disorders, particularly hypoglycemia, were the most common concerns in our patient cohort. Moreover, the data suggest that premature infants are at an increased risk for these endocrine issues.
The association of endocrine issues with preterm birth, low birth weight, and small size for gestational age is well-documented in the literature (3). In our study, we observed a similar pattern, with a substantial number of infants with endocrine problems being premature. The vulnerability of premature infants to endocrine challenges is a recognized aspect of neonatal care. These infants require tailored care plans to address their unique metabolic and endocrine needs, an essential consideration in NICU management.
Hypoglycemia is a condition closely linked to metabolic reserve constraints, frequently observed in infants with IUGR and those born to diabetic mothers (8). Additionally, infants with increased energy demands, such as those affected by perinatal asphyxia, sepsis, hypoxia, and hypothermia, are more susceptible to hypoglycemia. In our cohort, diabetic maternal infancy and prematurity emerged as primary contributors to transient neonatal hypoglycemia. A study by Harris et al. involving 514 infants aged 35 gestational weeks and above found that 98 (19%) of the infants at risk for hypoglycemia developed at least one hypoglycemic episode, based on criteria such as being SGA, LGA, infants of diabetic mothers, late preterm infants, and other clinical reasons with blood glucose levels below 47 mg/dL (9). Generally, hypoglycemia may occur in up to 5 - 15% of healthy term newborns, particularly within the first 24 to 48 hours after birth (8, 10). This rate increases to up to 51% in cases with risk factors. Our study identified glucose homeostasis disorders in 3.7% of our entire patient cohort and 24.7% of the subgroup presenting with endocrine issues. This discrepancy may reflect the differing risk profiles of the study populations and the inherent nature of the respective study designs. The timing and methodology of glucose screening, for which there is no universal consensus, likely contribute to the variability in reported incidences across studies (1). Moreover, the lack of a robust scientific justification for universally accepted blood glucose thresholds for neonatal hypoglycemia complicates the establishment of a standardized screening protocol.
Determining the precise prevalence of neonatal isolated HTT cases is challenging for several reasons. Firstly, some studies do not report FT4 levels, which complicates distinguishing mild congenital hypothyroidism from HTT. Secondly, there is a lack of consensus regarding the criteria used to define this condition (11). Due to the absence of agreed-upon TSH cutoff values, our study categorized cases with mildly elevated TSH levels despite normal FT4 levels as neonatal HTT. A detailed investigation during our study revealed that TSH elevation persisted beyond the postnatal twenty-first day, resulting in an actual prevalence of 3% for HTT among all hospitalized cases. A systematic review by Chiesa and Tellechea estimated the overall prevalence of neonatal HTT at 0.06% (11). The differences we highlighted may be due to methodological variations between studies, such as the lack of standardized reporting for FT4 levels and the absence of universally accepted TSH thresholds.
Hypothyroxinemia of prematurity, characterized by normal or low TSH and markedly low serum total T4 and FT4 levels, presents a diagnostic challenge, especially as these levels typically reach their lowest between postnatal days 10 and 14 (12, 13). This condition is more pronounced in infants with lower gestational ages and birth weights. Studies have shown that hypothyroxinemia of prematurity occurs in about 50% of premature and VLBW patients (14, 15). In our study, hypothyroxinemia of prematurity was detected in 3% of all premature cases observed. This disparity may stem from differences in laboratory methodologies for thyroid hormone quantification or from a cohort with fewer infants at the lower extremes of gestational age and weight.
Hypocalcemia is often associated with conditions such as prematurity, maternal diabetes, perinatal stress, sepsis, and IUGR (3, 16). Approximately one-third of premature infants and most VLBW infants exhibit low total serum calcium concentrations during the first two days after birth (16), though this rarely causes symptoms. In our study, prematurity was a primary cause of early neonatal hypocalcemia, aligning with existing literature (3, 17). We also found that hypomagnesemia was the most common cause of late neonatal hypocalcemia in our study. Common causes of late-onset hypocalcemia include excessive phosphate intake, hypomagnesemia, hypoparathyroidism, and vitamin D deficiency (3). Hypomagnesemia can impair parathyroid hormone (PTH) secretion and reduce peripheral response to PTH, leading to hypocalcemia. In this respect, our results are consistent with the literature.
Hypercalcemia, although rare and usually caused by various factors, is typically transient and asymptomatic (18). In our study, about half of the hypercalcemia cases were iatrogenic; two cases presented symptoms of constipation and polyuria. While it is difficult to make direct comparisons with the literature due to the lack of specific biomarker levels in our cases, low serum phosphorus levels were observed in a significant number of hypercalcemia cases.
In the neonatal context, magnesium imbalances, particularly hypomagnesemia, are frequently observed in newborns born to mothers with gestational diabetes and are often linked to abnormalities in magnesium and calcium metabolism (19). The exact incidence in neonates is not well-documented. However, neonates may be more susceptible to magnesium-related problems than other patient groups. Our study revealed that magnesium-related problems occurred in 1% of the cases studied, with hypomagnesemia being the most common concern. Moreover, neonatal hypermagnesemia, often an underrecognized condition, is frequently a consequence of therapeutic intravenous magnesium sulfate given to mothers with preeclampsia. Interestingly, in our cohort, the occurrence of neonatal hypermagnesemia was notable for its iatrogenic origin, arising independently of any maternal preeclampsia history. This suggests that other iatrogenic factors within the NICU setting may contribute to such imbalances, emphasizing the necessity for meticulous monitoring of magnesium therapy and its implications on neonatal magnesium levels.
Hypophosphatemia may occur within the first days of birth and can persist for many months. Typically, it has a nutritional basis and occurs most frequently in infants receiving low phosphorus intake from parenteral nutrition and in preterm infants fed with human milk (20). Our study identified hypophosphatemia in 3.3% of patients with endocrine problems, primarily due to receiving phosphorus-poor parenteral nutrition. This underscores the critical role of meticulous nutritional management in our neonatal intensive care settings, especially for infants at risk of hypophosphatemia.
5.1. Limitations and Future Studies
Our study has several limitations. First, it was conducted as a single-center study. The single-center design presents both strengths and challenges. While data derived from one center ensure consistency in diagnostic and treatment approaches, it may limit the generalizability of our findings across varied clinical settings. The study's generalizability is further limited by the unique demographic profile of the NICU's city, which does not reflect the broader national population. For example, in the city, the crude birth rate in 2021 was 8.5 (Türkiye's average is 12.8), and the total fertility rate was 1.32 (below Türkiye's average of 1.7) (21). The annual number of births for 2021 was 4 924. The rate of consanguineous marriage in this city is very low, at 2%, which is well below the national average (21). A pediatric endocrinologist and a neonatal intensive care specialist were present at the hospital for only three years of the five-year study period. This may have impacted the consistency of diagnoses and care, potentially introducing a degree of variability in our data. Additionally, the retrospective nature of this study introduces potential biases inherent to such a design. These include selection bias and the possibility of missing or incomplete data, which may affect the accuracy of our findings. These factors should be carefully considered when extrapolating our results to broader populations. Future multicenter, prospective studies are warranted to confirm and expand these results.
To enhance the findings of our study and improve clinical practice, we recommend that future research include multicenter studies to ensure broader data variability and enhanced generalizability. Establishing a consensus on diagnostic criteria for neonatal endocrine disorders is essential for enabling timely and accurate diagnoses. Long-term studies should explore the consequences of early recognition of these disorders, focusing on the development of advanced diagnostic techniques and the efficacy of various therapeutic interventions. Additionally, proactive strategies for the prevention of these conditions in high-risk groups should be developed, and targeted educational programs for NICU healthcare providers should be implemented. These steps may help to shape more effective and preventive neonatal endocrine care.
5.2. Conclusions
Given the findings of our study, it is crucial to develop more vigilant and preventive approaches to managing endocrine problems such as hypoglycemia, hypocalcemia, and thyroid disorders, which are noted in approximately 14.6% of NICU patients. Prematurity is a significant patient group in which endocrine problems occur. Establishing a consensus on the screening and management of neonatal hypothyroidism, including HTT, and hypoglycemia is critical. The observed hypophosphatemia, primarily due to phosphorus-poor parenteral nutrition, underscores the necessity for careful nutritional management. Similarly, the iatrogenic nature of many hypercalcemia and hypermagnesemia cases highlights the need for cautious use of fortified formulas and awareness of phosphate deficiency risks.
Understanding the prevalence and characteristics of endocrine disorders in NICU infants can aid in early diagnosis, intervention, and improved patient care. More research with larger and more diverse groups of newborns is needed to better understand these problems