1. Background
Idiopathic intracranial hypertension (IIH) is characterized by elevated cerebrospinal fluid pressure without any underlying brain abnormalities, space-occupying lesions, infections, or alterations in the brain parenchyma (1, 2). Typically, IIH presents with the primary symptom of headache, which can be particularly challenging to diagnose in younger children (3). As a result, diagnosing IIH in the pediatric population remains a significant challenge. The annual incidence of IIH in pediatric patients is estimated to be less than one case per 100 000 individuals, although this may underestimate its true prevalence due to diagnostic complexities (4). Given the potential complications of IIH, such as visual impairment and persistent symptoms (5), a more comprehensive understanding of this condition is essential (6).
Even after normalizing intracranial pressure, some patients experience persistent symptoms, including headache, depression, and anxiety (7, 8). Effective management of IIH necessitates a multidisciplinary approach, including pharmacotherapy, lifestyle adjustments, and, in some cases, neurosurgical interventions like shunt insertion and optic nerve sheath fenestration (9, 10). Notably, recurrence is a concern, particularly in children, with around one-third of patients experiencing it, sometimes within a year of treatment discontinuation (11).
Limited evidence exists on the predictive determinants of clinical and visual outcomes in children diagnosed with IIH. Previous research has primarily focused on investigating the natural progression, results, and variables that might predict the outcomes of childhood-onset IIH. However, these studies have been limited in sample size, with relatively small numbers of patients being included (12, 13). Additionally, several studies have primarily concentrated on examining the symptoms that manifest at the outset of IIH and the risk factors associated with the condition (14, 15).
Several previous studies have examined a restricted range of predicting factors for the visual result (16-18) or have incorporated a very brief duration of follow-up. The research findings indicate that the pubertal state is a significant factor in determining the manifestation and prognosis of diseases (4, 16).
2. Objectives
Additionally, it has been shown that obese children in puberty are less likely to have favorable visual results (18). Given the limited research in this area, our study aims to assess clinical presentations and risk factors of IIH in pediatric patients.
3. Methods
This study was a retrospective cohort protocol conducted on patients admitted to hospitals with a final diagnosis of IIH. Clinical information was extracted from pediatric IIH patients in two main referral children's hospitals of Shahid Beheshti University of Medical Science (SBMU) in Tehran, the capital of Iran. Subsequently, the patients were contacted for follow-up regarding their symptoms. Parents of the patients provided consent for participation in the study and the potential publication of their clinical data while ensuring their anonymity during interviews. The study received approval from the Ethics in Biomedical Research Committee of Shahid Beheshti University of Medical Sciences under the code IR.SBMU.MSP.REC.1398.1038.
3.1. Study Setting and Participants
We utilized medical records of patients admitted to referral children's hospitals between 2013 and 2021. These patients were then contacted and followed up as part of this study. Inclusion criteria encompassed patients under 18 years of age, diagnosed with IIH, and admitted to our hospitals with willingness, complete documentation, and successful follow-up interviews. The diagnosis of IIH was confirmed by pediatric neurologists and ophthalmologist consultants according to Dandy criteria and the 2013 revision proposed by Friedman et al. (19, 20). Exclusion criteria comprised patients older than 18 years, incomplete documentation, unavailability during follow-up, non-admission, and parental unwillingness to participate.
3.2. Data Sources and Variables
Clinical and demographic information, including sex, weight, and presenting signs and symptoms, were extracted from medical records. Thorough ophthalmologic examinations were conducted and documented for all patients. For our retrospective study, we primarily collected data by reviewing patients' records and conducting interviews. This approach became necessary due to the limitations imposed during the COVID-19 pandemic. The lack of in-person access made conducting thorough clinical examinations difficult. Unfortunately, this restriction hindered the ability to monitor vision progress effectively as it took time to obtain the necessary cooperation. Consequently, we assessed vision by analyzing the data obtained from acuity and visual field tests and a questionnaire that inquired about any recent or ongoing vision issues such as blurred vision, diplopia, photophobia, and strabismus. Additionally, in this study, the assessment of headaches was done by completing questionnaires designed by the ICHD-3 criteria published in the Cephalalgia journal for evaluating headaches in IIH patients (21). It is important to note that recurrent symptoms mirrored the initial presentation, encompassing headache, nausea, vomiting, and confirmed papillary edema upon examination.
Lumbar puncture records were assessed for the opening pressure observed. Idiopathic intracranial hypertension diagnosis followed the criteria suggested by Friedman et al. (3, 20). Therefore, in the present study, according to the revised diagnostic criteria used to confirm IIH, the different opening pressure thresholds for diagnosing IIH in the examined children were 28 cmH2O, and in the condition that the child was not under sedation and not obese, 25 cmH2O was considered (20, 22, 23). Moreover, it is crucial to highlight that we consistently measured the mentioned metric at least twice in our study patients.
Cerebrospinal fluid (CSF) opening pressure was measured using a standardized procedure. The lumbar puncture was performed with the patient in the lateral decubitus position. A sterile, disposable spinal needle was carefully inserted into the subarachnoid space between the L3/L4 or L4/L5 intervertebral spaces, guided by anatomical landmarks. Prior to the procedure, the skin was sterilized, and local anesthesia or sedation was administered.
The CSF opening pressure was measured using a manometer connected to the spinal needle. The manometer was calibrated before each measurement to ensure accuracy. The procedure was performed by experienced clinicians in a controlled environment, adhering to aseptic techniques to minimize the risk of contamination.
This standardized approach was consistently employed across all study participants to ensure uniformity in CSF opening pressure measurements. We used local anesthesia for cooperative pediatric patients, while those who were uncooperative underwent the measurement while sedated in the operating room. Additionally, all patients underwent brain imaging via MRI or CT scan to rule out alternative conditions such as brain tumors.
3.3. Statistical Analyses
Data were entered into IBM SPSS version 26.0. Quantitative variables were reported as means and standard deviations, while qualitative variables were presented as frequencies and percentages. Independent-samples t-test and chi-square tests were utilized to compare results between two groups. In case of non-normal distribution of the variables, the Mann-Whitney test was used to compare two groups. Binary logistic regression analysis was employed to create a prognostic tool for predicting the development of IIH complications.
4. Results
A total of 89 patients were identified within the study period. However, three patients had incomplete documentation and remained unreachable through the contact details provided in their records. Additionally, five patients opted not to participate in the study. Consequently, the study included 81 participants, out of which 43 (53.1%) were male and 38 (46.9%) were female. The patients' average age was 13.6 ± 4.4 years, while the mean age at the time of diagnosis was 10.3 ± 4.2 years. The mean weight percentile for patients at the time of diagnosis, as per WHO growth curves, was 41.9 ± 26.8. In our study, 2 patients had optic nerve fenestration, and 5 patients underwent shunting. The median lumbar CSF opening pressure in our patient was 39 cmH2O, with an interquartile range of 23.5 - 60 cmH2O, also the lowest and the highest recorded intracranial pressure being 7 and 130 cmH2O, respectively. The duration of patient follow-up was 3.1 ± 1.5 years.
To assess the normality of weight percentiles, the Kolmogorov-Smirnov test was employed, yielding a P-value of < 0.001, indicating non-normal distribution of the data. Despite this, the data exhibited a relatively symmetrical skewness of 0.273. The largest proportion of weight percentile values fell within the < 10% range (20 patients). Also, a significant number of underweight patients were identified in our study (14 patients with weight percentiles < 3). Moreover, 19% of patients met the criteria for being overweight or obese, which is higher than the regional and national averages. Table 1 provides an overview of the patients' demographic information. Additionally, Table 1 presents a comprehensive summary of both demographic and clinical particulars of the patients.
| Variables | Values |
|---|---|
| Age (y) | 13.6 ± 4.4 |
| Weight percentile | 41.9 ± 26.8 |
| Lumbar CSF opening pressure (cmH2O) | 42.9 ± 25.2 |
| Sex | |
| Male | 43 (53.1) |
| Female | 38 (46.9) |
| Clinical signs and symptoms | |
| Headache | 69 (85.2) |
| Nausea/vomiting | 36 (44.4) |
| Strabismus | 23 (28.4) |
| Visual impairment | 38 (46.9) |
| Diplopia | 41 (50.6) |
| Eye pain | 7 (8.6) |
| Photophobia | 8 (9.9) |
| Papilledema | 64 (79.0) |
| Decreased visual acuity | 3 (3.7) |
| Impaired visual field | 2 (2.5) |
| Outcome | |
| Recurrent disease | 13 (16.0) |
| Persistent headache | 13 (16.0) |
| Persistent visual problems | 12 (14.8) |
| Medications | |
| Acetazolamide | 69 (85.2) |
| Topiramate | 9 (11.1) |
| Corticosteroids | 26 (32.1) |
| Antiepileptics | 6 (15.8) |
| Mannitol | 5 (13.2) |
z Abbreviation: CSF; cerebrospinal fluid.
a Values are expressed as No. (%) or mean ± SD.
Out of the total participants, 13 (16.0%) experienced recurrent episodes of IIH, while 68 (84.0%) did not encounter such recurrences. The most prevalent clinical symptom reported by patients was headache (85.2%), followed by diplopia (50.6%), visual impairment (46.9%), nausea and/or vomiting (44.4%), strabismus (28.4%), photophobia (9.9%), and ophthalmodynia (8.6%). Papilledema was the predominant clinical sign observed among patients (79.0%). Furthermore, 2 (2.5%) patients also reported experiencing visual field defects.
In terms of medical history, three (3.7%) patients had a previous diagnosis of attention deficit hyperactivity disorder (ADHD). Among the patient cohort, 71 patients (87.7%) were managed medically, while others required interventional procedures, including ventriculoperitoneal shunt insertion, shunt replacements, therapeutic lumbar punctures, or even optic nerve sheath fenestration. Among the treatments, 70 patients received acetazolamide, with this being the sole medication for 35 (43.2%) patients. In addition to acetazolamide, other medical agents such as topiramate, dexamethasone, methyl prednisolone, and mannitol were employed for treatment in other patients. Patients who received acetazolamide as monotherapy exhibited lower lumbar puncture opening pressure compared to those who needed combination therapy. However, this difference was not statistically significant (34.37 ± 29.10 cmH2O vs. 44.52 ± 26.02 cmH2O, respectively, P = 0.163).
The relationship between sex and various signs, symptoms, and outcomes of the disease was assessed using the chi-square test and Fisher’s exact test. The summarized results of these tests are presented in Table 2.
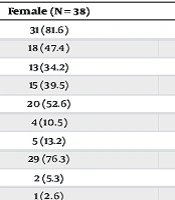
| Variables | Male (N = 43) | Female (N = 38) | P-Value |
|---|---|---|---|
| Headache | 38 (88.4) | 31 (81.6) | 0.390 |
| Nausea and/or vomiting | 18 (41.9) | 18 (47.4) | 0.619 |
| Strabismus | 10 (23.3) | 13 (34.2) | 0.275 |
| Visual impairment | 23 (53.5) | 15 (39.5) | 0.207 |
| Diplopia | 21 (48.8) | 20 (52.6) | 0.733 |
| Eye pain | 3 (7.0) | 4 (10.5) | 0.701 |
| Photophobia | 3 (7.0) | 5 (13.2) | 0.464 |
| Papilledema | 35 (81.4) | 29 (76.3) | 0.575 |
| Decreased visual acuity | 1 (2.3) | 2 (5.3) | 0.598 |
| Impaired visual field | 1 (2.3) | 1 (2.6) | > 0.999 |
| Recurrence | 11 (25.6) | 2 (5.3) | 0.013 |
| Persistent headache | 10 (23.3) | 3 (7.9) | 0.060 |
| Persistent visual problems | 6 (14.0) | 6 (15.8) | 0.816 |
a Values are expressed as No. (%).
b A P-value below 0.05 is indicative of statistical significance.
The results showed that recurrence was more frequent in male patients, but the signs and symptoms of the patients did not differ between male and female patients. The outcome of the disease regarding persistent headache and visual problems also did not differ between male and female patients. Chi-square test and Fisher’s exact test were also used to assess the relationship between recurrence and different signs, symptoms, and outcomes of this disease. Most of the patients who experienced recurrent episodes were male, which was significantly different (25.6% of males and 5.3% of females experienced recurrence, P-value = 0.013) (see Table 2). Patients with and without recurrent episodes differed significantly regarding presentation with strabismus (0% in recurrent disease vs. 33.82% in non-recurrent disease, P-value = 0.013). The results of these comparisons are summarized in Table 3.
| Variables | Recurrence (N = 13) | No Recurrence (N = 68) | P-Value b |
|---|---|---|---|
| Sex | 0.013 | ||
| Male | 11 (84.6) | 32 (47.1) | |
| Female | 2 (15.4) | 36 (52.9) | |
| Headache | 13 (100) | 56 (82.4) | 0.101 |
| Nausea and/or vomiting | 6 (46.2) | 30 (44.1) | 0.892 |
| Strabismus | 0 (0.0) | 23 (33.8) | 0.013 |
| Visual impairment | 9 (69.2) | 29 (42.6) | 0.078 |
| Diplopia | 4 (30.8) | 37 (54.4) | 0.118 |
| Eye pain | 2 (15.4) | 5 (7.4) | 0.345 |
| Photophobia | 3 (23.1) | 5 (7.4) | 0.082 |
| Papilledema | 11 (84.6) | 53 (77.9) | 0.588 |
| Decreased visual acuity | 0 (0.0) | 3 (4.4) | > 0.999 |
| Impaired visual field | 0 (0.0) | 2 (2.9) | > 0.999 |
| Persistent headache | 4 (30.8) | 9 (13.2) | 0.115 |
| Persistent visual problems | 3 (23.1) | 9 (13.2) | 0.360 |
a Values are expressed as No. (%).
b A P-value below 0.05 is indicative of statistical significance.
The mean weight percentile for patients was found to be 41.6 ± 36.1 for males and 42.4 ±3 8.4 for females, demonstrating no statistically significant difference (P = 0.975). Furthermore, despite the slightly higher mean intracranial pressure among female patients compared to male patients (34.82 ± 22.41 cmH2O for boys and 44.38 ± 31.14 cmH2O for girls), this difference was not statistically significant (P = 0.141). To assess the connection between weight percentile and lumbar puncture opening pressure, a Pearson’s correlation test was performed, indicating a non-significant positive correlation (P = 0.094, r = 0.220). Interestingly, patients presenting with visual impairment and impaired visual fields were comparatively older than those without these symptoms (P = 0.005 and P = 0.030, respectively). Conversely, patients experiencing persistent headaches exhibited higher weight percentiles (61.6 ± 3 6.2 vs. 38.3 ± 35.7, P = 0.027). Table 4 summarizes the comparison of age, weight percentile, and CSF opening pressure based on clinical presentation and outcome of the disease.
| Variables | Age | P-Value | Weight Percentile | P-Value c | CSF Opening Pressure | P-Value d |
|---|---|---|---|---|---|---|
| Sex | 0.154 | 0.975 | 0.141 | |||
| Male | 9.81 ± 3.65 | 41.59 ± 36.10 | 34.82 ± 22.41 | |||
| Female | 10.89 ± 4.77 | 42.41 ± 38.41 | 44.38 ± 31.14 | |||
| Headache | 0.304 | 0.665 | 0.887 | |||
| No | 8.53 ± 5.88 | 40.71 ± 42.68 | 37.75 ± 29.77 | |||
| Yes | 10.63 ± 3.83 | 42.13 ± 36.10 | 39.38 ± 26.85 | |||
| Nausea/vomiting | 0.402 | 0.903 | ||||
| No | 10.06 ± 4.22 | 0.492 | 38.21 ± 34.96 | 38.82 ± 23.51 | ||
| Yes | 10.64 ± 4.26 | 50.83 ± 40.38 | 39.63 ± 30.98 | |||
| Strabismus | 0.071 | 0.322 | 0.891 | |||
| No | 9.87 ± 4.43 | 38.21 ± 34.96 | 38.85 ± 24.93 | |||
| Yes | 11.46 ± 3.46 | 50.83 ± 40.38 | 39.88 ± 31.34 | |||
| Visual impairment | 0.005 | 0.539 | 0.614 | |||
| No | 9.11 ± 3.77 | 39.82 ± 38.00 | 40.78 ± 29.98 | |||
| Yes | 11.68 ± 4.33 | 44.75 ± 35.57 | 37.51 ± 23.72 | |||
| Diplopia | 0.143 | 0.511 | 0.119 | |||
| No | 9.46 ± 4.81 | 38.66 ± 36.79 | 33.68 ± 23.26 | |||
| Yes | 11.16 ± 3.42 | 45.00 ± 37.06 | 43.57 ± 29.15 | |||
| Eye pain | 0.649 | 0.891 | 0.091 | |||
| No | 10.21 ± 4.31 | 42.27 ± 36.76 | 36.75 ± 25.29 | |||
| Yes | 11.43 ± 3.26 | 37.54 ± 41.29 | 65.17 ± 33.15 | |||
| Photophobia | 0.605 | 0.346 | 0.390 | |||
| No | 10.20 ± 3.99 | 43.13 ± 36.50 | 38.11 ± 26.90 | |||
| Yes | 11.38 ± 6.21 | 26.73 ± 41.40 | 47.54 ± 27.86 | |||
| Papilledema | 0.740 | 0.176 | 0.461 | |||
| No | 9.73 ± 4.74 | 32.42 ± 39.02 | 44.93 ± 33.24 | |||
| Yes | 10.48 ± 4.10 | 44.85 ± 35.96 | 37.76 ± 25.33 | |||
| Decreased visual acuity | 0.339 | > 0.999 | 0.787 | |||
| No | 10.23 ± 4.25 | 42.03 ± 37.06 | 38.81 ± 26.48 | |||
| Yes | 12.67 ± 3.26 | 34.83 ± N/A | 52.00 ± 53.74 | |||
| Impaired visual field | 0.030 | 0.824 | 0.521 | |||
| No | 10.18 ± 4.18 | 42.26 ± 36.97 | 38.90 ± 27.26 | |||
| Yes | 16.00 ± 0.00 | 19.77 ± N/A | 49.00 ± 15.56 | |||
| Recurrence | 0.289 | 0.900 | 0.286 | |||
| No | 10.04 ± 4.25 | 37.29 ± 35.68 | 37.87 ± 28.05 | |||
| Yes | 11.77 ± 3.90 | 61.55 ± 36.25 | 45.58 ± 20.81 | |||
| Persistent headache | 0.380 | 0.027 | 0.082 | |||
| No | 10.41 ± 4.53 | 38.29 ± 35.68 | 40.90 ± 28.36 | |||
| Yes | 9.85 ± 2.09 | 61.55 ± 36.25 | 30.00 ± 15.74 | |||
| Persistent visual problem | 0.774 | 0.106 | 0.844 | |||
| No | 10.33 ± 4.25 | 38.66 ± 36.41 | 39.48 ± 27.00 | |||
| Yes | 10.25 ± 2.96 | 58.84 ± 35.61 | 37.64 ± 28.104 |
a Values are expressed as mean ± SD.
b A P-value below 0.05 is indicative of statistical significance.
c This P-value serves as an indicator of the statistical significance of the observed disparity in weight percentile between two distinct groups for instance differences between genders, presence of headache, or other relevant groupings.
d This P-value serves as an indicator of the statistical significance of the observed disparity in CSF Opening Pressure between two distinct groups for instance differences between genders, presence of headache, or other relevant groupings.
Even after receiving treatment at the hospital, nine patients (11.1%) continued to experience headaches, while another nine patients (11.1%) still faced visual impairments. In an endeavor to appraise the effectiveness of our data-based model in prognosticating the persistence of headache and visual problems, a binary logistic regression test was conducted.
For the model predicting persistent headache, the Omnibus tests of model coefficients yielded a P-value of 0.261. This outcome implies that our model lacked the ability to predict the persistence of headaches accurately. On the contrary, when a binary logistic regression was performed for predicting the persistence of visual problems, the Omnibus tests of model coefficients reported a P-value of 0.007. Notably, the Nagelkerke R2 value for our model stood at 0.674. The predictive accuracy of our model for future visual problems was 93.8% for patients without persistent visual issues and 54.5% for patients with such problems, resulting in an overall accuracy of 86.4%. A comprehensive outline of the model for predicting future visual problems is presented in Table 5.
| Variables | B | SE | Wald | df | P-Value | Exp (B) |
|---|---|---|---|---|---|---|
| Age at time of diagnosis | -0.234 | 0.257 | 0.832 | 1 | 0.362 | 0.791 |
| Sex | 1.837 | 1.500 | 1.500 | 1 | 0.221 | 6.278 |
| Weight percentile | 0.040 | 0.020 | 4.022 | 1 | 0.045 | 1.040 |
| Nausea and/or vomiting | 0.613 | 1.261 | 0.236 | 1 | 0.627 | 1.846 |
| Strabismus | 2.256 | 1.923 | 1.376 | 1 | 0.241 | 9.548 |
| Visual impairment | -0.199 | 1.213 | 0.027 | 1 | 0.870 | 0.820 |
| Eye pain | -20.436 | 15303.752 | 0.000 | 1 | 0.999 | 0.000 |
| Photophobia | -18.604 | 16371.180 | 0.000 | 1 | 0.999 | 0.000 |
| Impaired visual field | -12.976 | 40192.970 | 0.000 | 1 | > 0.999 | 0.000 |
| Intracranial pressure | 0.015 | 0.025 | 0.370 | 1 | 0.543 | 1.016 |
| Recurrence | 5.089 | 2.379 | 4.574 | 1 | 0.032 | 162.206 |
| Headache | 20.893 | 13202.473 | 0.000 | 1 | > 0.999 | 1184703310 |
| Diplopia | 2.005 | 1.727 | 1.349 | 1 | 0.245 | 7.429 |
| Papilledema | 20.336 | 8303.748 | 0.000 | 1 | 0.998 | 679230324 |
| Decreased visual acuity | 27.567 | 40192.970 | 0.000 | 1 | > 0.999 | 9.376E + 11 |
aA P-value below 0.05 is indicative of statistical significance.
The results show that only weight percentile and recurrence were significant predictors for future visual problems, with recurrence having stronger predictive power.
5. Discussion
In our study, we investigated 81 pediatric patients diagnosed with IIH in Tehran, Iran, to unravel the clinical presentations, risk factors, and prognostic indicators of this condition in the pediatric population. The study demonstrated nearly even male-to-female distribution, according to the average age at diagnosis and the mean weight percentile of patients, reflecting demographic diversity. Comprehensive symptom analysis revealed that headaches were predominant, followed by diplopia, visual impairment, nausea and/or vomiting, and strabismus. Papilledema was the most common clinical sign, emphasizing the importance of early diagnosis. Our study also explored various treatments, including medical management and surgery. An intriguing finding was the comparable impact of acetazolamide, whether used as monotherapy or in combination, on lumbar puncture opening pressure. Moreover, gender and recurrent episodes were noted as influencing IIH's clinical course, with males showing a higher recurrence disposition. Interactions between clinical symptoms, weight percentiles, and lumbar puncture opening pressures provided insights into pediatric IIH complexities. Significantly, our data-driven model accurately predicted the persistence of visual problems. Weight percentile and recurrence emerged as significant predictors for future visual problems, contributing to a deeper understanding of pediatric IIH. These findings have significant implications for improved patient management and informed clinical decision-making.
In a study by Yamamoto et al., the authors evaluated 165 children with IIH over a 27-year period. They found that patients predominantly presented with headache, visual impairments, and nausea and/or vomiting (24). Our study's results align with theirs, demonstrating similar findings. Additionally, they observed a change in the male-to-female ratio during the prepubertal and postpubertal periods. While our study didn't specifically address patients' puberty status, we noted that among patients younger than 12 years old, approximately 60.0% were male, while patients older than 12 years old had about 41.9% male representation. This similarity in the gender trend across studies is notable.
Paley et al. explored the association between obesity and pediatric Secondary IIH, revealing a 2.47 times higher prevalence of obesity in patients compared to the normal population, suggesting that obesity might be a risk factor for Secondary IIH (25). However, our study showcased a notable proportion of underweight patients (14 patients with weight percentiles < 3). Furthermore, 19% of patients were classified as overweight or obese, surpassing national and regional averages. These results imply that both ends of the weight percentile spectrum carry higher risks for pediatric IIH (26).
Tovia et al. reported a response rate of 76.6% to acetazolamide in pediatric patients with IIH (27). In our study, 69 patients initially underwent acetazolamide treatment, with 35 patients not requiring additional therapy. As such, our study reported a similar rate of 50.72%. The slightly higher lumbar puncture opening pressure and wider variance among our patients suggest that differences in diagnosis timing or disease severity may account for our results. This is especially true for patients needing combination therapy, who exhibited higher intracranial pressures.
Remarkably, 3.7% of our patients had a history of ADHD, which is lower than the prevalence of this disorder reported in a recent meta-analysis (28). Given the lack of prior studies exploring the link between ADHD and pediatric IIH, further research is needed to investigate this possible relationship.
Another study conducted by Senderowich et al. examined a cohort of 97 patients and placed particular emphasis on the adverse progression of IIH, the persistence of headaches, and the suboptimal visual results (29). The researchers emphasized variables such as gender and illness recurrence, identifying them as significant indicators of unfavorable visual outcomes. In their investigation, 29% of patients exhibited persistent headaches at the last follow-up. Importantly, Senderowich et al.'s work emphasizes the complexity of distinguishing between chronic headache syndromes and IIH recurrence, given the overlapping clinical features and comorbidity with chronic migraine, especially in cases without papilledema. The results of their study highlight the probable existence of a unique condition among those with chronic headaches, which may necessitate a modified treatment strategy. In our study, we echo Senderowich et al.'s emphasis on certain variables, such as gender and illness recurrence, as significant indicators of unfavorable visual outcomes in pediatric IIH. Our research focuses on examining the clinical manifestations and potential risk factors associated with pediatric IIH. In conjunction with our investigation, that study provides additional insights into the difficulties presented by an unfavorable disease progression and the distinctive characteristics of chronic headaches experienced by this specific group of patients. The collective insights from both studies contribute to an enriched understanding of the complexities surrounding disease progression, particularly in cases involving persistent headaches, thus supporting the development of tailored treatment strategies for pediatric IIH.
Numerous studies have explored risk factors for recurrence or persistent symptoms in IIH patients. Prior literature has shown that lumbar puncture opening pressure isn't associated with an increased risk of recurrence. However, papilledema and longer symptom durations are linked to a higher risk of recurrence. Additionally, extended treatment duration correlates with a reduced recurrence risk. Studies investigating the connection between sex, age at diagnosis, and recurrence risk have produced clear results (11, 30-34). Our study found that recurrence was less frequent in female patients and those with strabismus, implying that female sex might act as protective factors. However, more research is necessary to verify this relationship.
In our study, intracranial pressure and weight percentile didn't differ significantly between patients with and without recurrence. Consequently, these two factors are not reliable predictors of recurrence. Bhalla et al. similarly found that obesity or underweight status weren't risk factors for recurrence (33).
Given that the most concerning outcome of IIH is long-standing visual impairments and blindness, creating a prognostic model for this sequela is of paramount importance (35). Sorensen et al. discovered that persistent visual problems can persist even after intracranial hypertension has resolved (36). Alfonso et al. evaluated 50 adult IIH patients and concluded that a delayed interval from presentation to diagnosis, higher maximal intracranial pressure, and hypertension were linked to severe vision loss (37). In contrast, our study identified weight percentile and recurrence history as significant predictors of persistent visual impairment. The variation in results between the two studies might be attributed to differences in the studied populations and the sex ratios. Alfonso et al.'s study predominantly featured female participants, limiting the generalizability of their results to other populations (37).
5.1. Limitations
The present investigation deals with limitations arising from the infrequency of the cases and the difficulties associated with reaching certain patient groups. Including patients who are under continuous medical follow-up and the challenges associated with accessing specific instances constitute a potential selection bias, adding to the study's limitations. Due to limitations in our study, such as a retrospective design covering an extended period, it is susceptible to missing information from medical records.
5.2. Conclusions
In this study, we evaluated the presentations of Pseudotumor cerebri in children and found that headache and diplopia did not differ between girls and boys. Additionally, the study showed that female sex served as a protective factor against recurrence, and the recurrence of IIH was significantly lower in patients with strabismus. Weight percentile and recurrence were predictive factors for persistent visual problems, but our model could not predict persistent headache.