1. Introduction
Moyamoya disease (MMD) is a progressive steno-occlusive arteriopathy of unknown origin. It is characterized by progressive stenosis and eventual occlusion of the distal intracranial internal carotid arteries (ICAs), as well as the proximal branches of the anterior cerebral artery (ACA) and the middle cerebral artery (MCA). Perfusion of the brain distal to the occlusion is maintained through the development of collateral blood vessels from the leptomeninges and both external and intracranial ICAs. In affected children, autoregulation of cerebral blood flow (CBF) is compromised, increasing the risk of cerebral ischemia (1). Neurological manifestations of the disease can range from transient ischemic attacks (TIA), slow cognitive decline, headaches, dizziness, seizures, monoparesis, hemiparesis, visual and sensory impairments, to cerebral infarction. Diagnosis of MMD follows specific guidelines and is typically confirmed through characteristic cerebral angiography and magnetic resonance imaging findings (2). The therapeutic goal is to reduce the risk of repeated strokes and further brain damage. Surgical revascularization helps prevent repeated strokes by enhancing blood flow to the affected cerebral hemispheres and can be achieved through direct cerebral bypass, indirect bypass, or a combination of both. Indirect revascularization improves cerebral perfusion by attaching vascularized grafts to the cortical surface to facilitate neoangiogenesis (3, 4). After revascularization, increased blood flow is expected in the affected brain areas. Although the success rate of indirect revascularization in pediatric patients is 95%, careful management is required as neoangiogenesis depends on time (5, 6). We present the anesthetic management of a pediatric patient scheduled for a postoperative follow-up endocranial MRI one year after revascularization.
2. Case Presentation
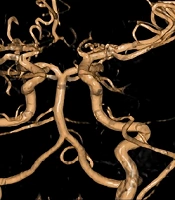
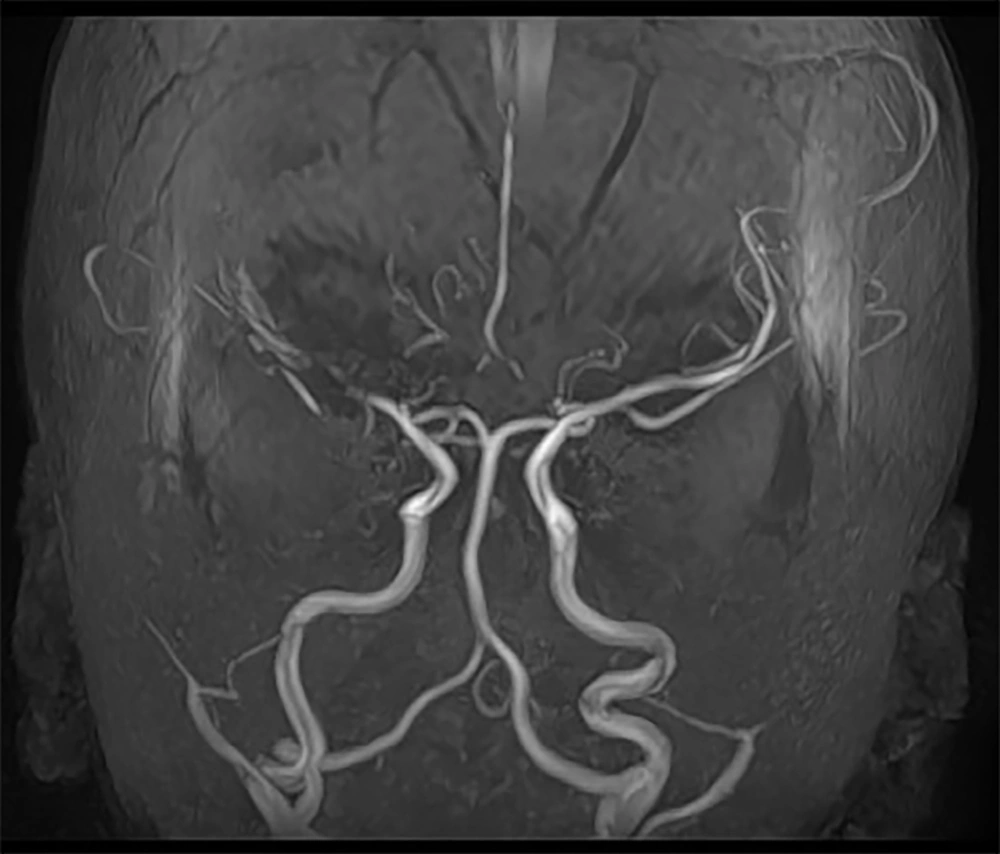
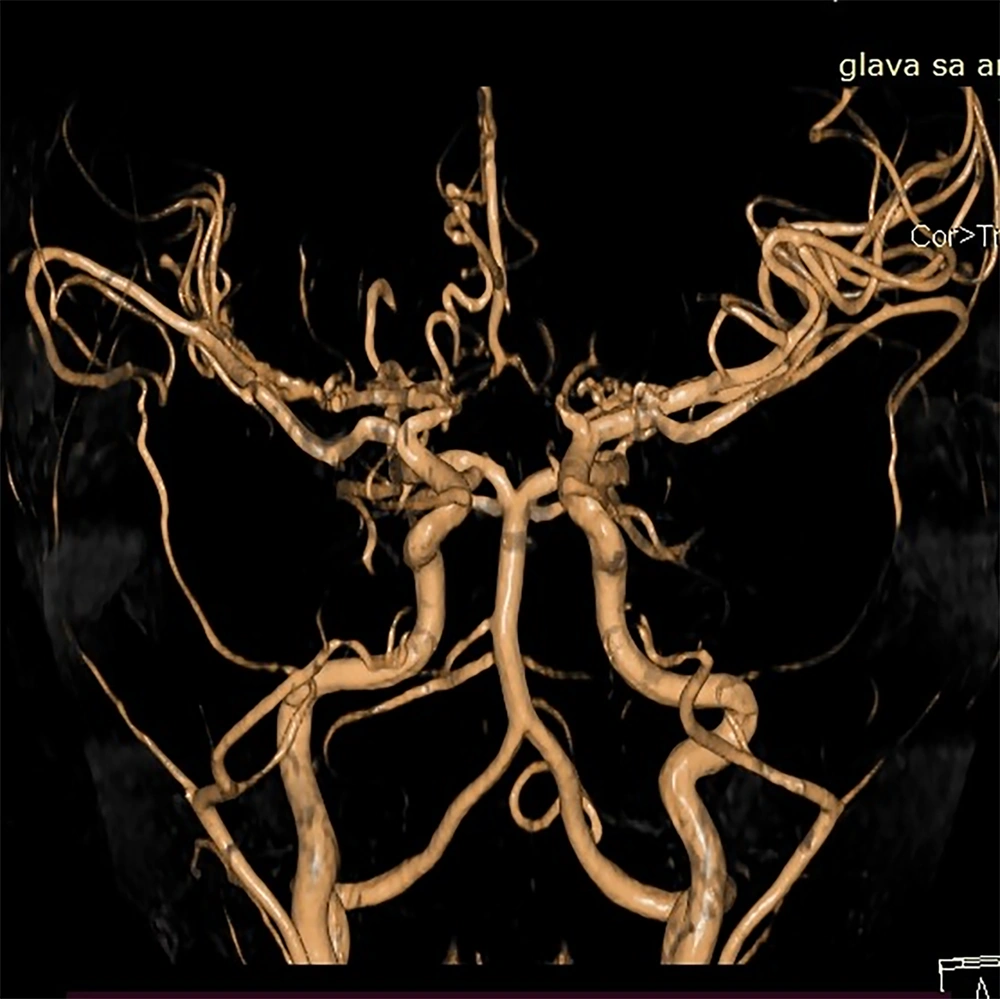
Our patient, a six-year-old boy, was diagnosed with moyamoya angiopathy at the age of four years and three months. The disease initially manifested as left-sided hemiparesis due to ischemia in the vascularization areas of the MCA and ACA. Three months after the initial symptoms, the boy developed aphasia due to a transient ischemic attack (TIA). Digital subtraction angiography showed subocclusive stenosis of the MCA, a hypoplastic proximal part of the ACA, and developed pial collateral circulation from both choroid arteries and the posterior cerebral artery (Figure 1). Seven months post-diagnosis, indirect revascularization procedures were performed—encephalo-duro-arterio-synangiosis (EDAS), encephalo-galeo-periosteal-synangiosis (EGPS), and encephalo-myo-synangiosis (EMS). Both anesthesia and surgery proceeded without incident.
In addition to angiopathy, the boy is at high risk for thrombosis as he is homozygous for the MTHFR 677CT gene and has elevated homocysteine levels. He is also heterozygous for the PAI-1 gene. He is prescribed a daily regimen of 75 mg aspirin to prevent thrombosis. Additionally, he is allergic to atropine, which manifests as a skin reaction.
The preoperative exam showed a fever-free, communicative, and very curious six-year-old boy weighing 19 kg and measuring 112 cm in height. His vital signs were normal for his age, with a blood pressure of 102/67 mmHg, a heart rate of 92 beats per minute, a respiratory rate of 17 breaths per minute, and an SpO2 of 97% on room air. Neurological examination revealed very subtle weakness in the left leg and arm. Blood count, urea, and creatinine levels were normal. Preoperative fasting was advised according to ESPA guidelines (7). He was premedicated 30 minutes before induction with midazolam syrup (0.5 mg/kg), cetirizine syrup (2.5 mg), and methylprednisolone (1 mg/kg) IV.
The patient was cooperative, allowing for a smooth inhalation induction with sevoflurane at 2 vo l%, reaching a Ramsay score of 5 in three minutes. Sedation was maintained with 1 vo l% sevoflurane in a 40:60 air/oxygen mixture via nasal cannula, with the patient breathing spontaneously at an average rate of 16 breaths per minute. Average blood pressure was 92/55 mmHg and heart rate was 98 beats per minute. During sedation, intravascular volume was maintained with normal saline at 90 mL/h. The procedure lasted 60 minutes. Two minutes before the end of imaging, sevoflurane was discontinued, and a Stewart score of 6 was reached within two minutes. There were no complications related to sedation, and the boy was discharged home two hours after the procedure.
3. Discussion
Moyamoya angiopathy, first identified in the Japanese population, has been documented across all races over the past seven decades, though it remains rare (1, 8, 9). There is currently no curative treatment for this progressive disease. Revascularization procedures are the only therapeutic options that enable affected children to lead a normal life. These procedures can be categorized as either direct or indirect. Direct revascularization involves creating anastomoses between scalp and cerebral cortical arteries, a technique that is challenging to perform in children. Conversely, indirect revascularization, which has a success rate of up to 95% in pediatric patients, involves placing galea, muscles, periosteum, or a combination thereof onto the surface of the ischemic brain, allowing blood vessels to grow from the donor tissue into the brain (5, 6). The specific procedure is named according to the tissue used, such as encephaloduroarteriosynangiosis, encephalogaleoperiosteosynangiosis, encephalomyosynangiosis or encephaloduroarteriomyosynangiosis (10).
Current data indicate a variation in the incidence of complications post-revascularization (10% - 30%), with most authors agreeing that neurological complications are more prevalent in children under six and within the first two postoperative years (11-13). These factors place operated children in a high-risk category during anesthesia due to potential anesthesia-induced changes in CBF in already compromised cerebral vasculature. Our patient was particularly at high risk due to his age, being within the critical two-year postoperative window, and having a genetic predisposition for thrombosis.
Cerebral blood flow is theoretically explained by Poiseuille’s law, which states that CBF is determined by cerebral perfusion pressure (CPP) and cerebral vascular resistance (CVR), with the relationship described by the equation CBF = ΔCPP/ΔCVR. However, the brain must be understood as a biological system where blood vessels do not simply act as pipes, and blood flow can be turbulent, especially at vessel branching points. Blood also possesses specific rheological characteristics. Factors that influence CPP and CVR, such as changes in hematocrit, hemoglobin, and clotting activity, can impact CBF. Intraoperative conditions like hypovolemia and hypotension, significant alterations in hematocrit or hemoconcentration, hypercarbia or hypocarbia, and a history of stroke or TIA can increase the risk of ischemic episodes.
To avoid such undesirable scenarios, it is crucial to maintain normotension, normoventilation, normoxia, and normocarbia during anesthesia or deep sedation. Deep sedation is a drug-induced depression of consciousness where the child cannot be aroused by verbal commands or light tactile stimulation but responds purposefully to pain or repeated stimulation. The ability to maintain spontaneous ventilation and airway function is preserved, although some children, especially those with comorbidities such as craniofacial anomalies, obesity, or neurological impairments, may require support in maintaining their airway. Cardiovascular function is generally maintained. Since MRI is a painless diagnostic procedure that requires the child to remain motionless for successful imaging, deep sedation is an appropriate technique for this context. Our goals were to prevent movement during MRI, avoid both hyperventilation and hypoventilation, maintain mean arterial pressure in the autoregulatory range above 50 mmHg, and preserve intravascular volume. Agitation and crying can induce hyperventilation, which further promotes cerebral hypocapnic vasoconstriction and vasospasm. Conversely, hypoventilation can cause regional cerebral vasodilation and the CBF steal phenomenon. Hyperventilation can be prevented by effective preanesthetic psychological preparation and premedication.
Fortunately, our patient had undergone thorough psychological preparation by his parents, making him very cooperative. We administered midazolam syrup to mitigate potential separation anxiety. Methylprednisolone and cetirizine were given as a precaution against possible reactions to atropine, as glycopyrrolate was not available at the time. A key aspect of our plan was to achieve a stable level of deep sedation while maintaining spontaneous ventilation to avoid hypoventilation and ensure normotension, thereby preventing unwanted sympathetic suppression.
To achieve our goals, we opted for deep sedation using sevoflurane. This inhalation anesthetic is a halogenated fluorocarbon with rapid onset and recovery, allowing quick adjustment of anesthetic depth. Widely used for decades in pediatric anesthesia, sevoflurane has a pleasant smell, is easily administered via face mask or nasal cannula, and its vapor is compatible with every anesthesia machine, including those designed for use in magnetic imaging suites. Its uptake and elimination are directly proportional to the patient's respiratory rate. Sevoflurane's negative feedback on respiratory drive protects against anesthetic overdose (14, 15), and it is eliminated almost entirely (99%) by the lungs, facilitating rapid recovery suitable for outpatient procedures.
Clinically administered doses of up to 1 MAC of sevoflurane typically do not impact systemic hemodynamics significantly; it does not cause myocardial depression or hypotension in children with healthy hearts and preserved intravascular volume, nor does it affect cerebral autoregulation and cerebrovascular reactivity (16). Once we observed a sluggish response to light touch, we reduced the inhaled concentration of sevoflurane from 2 vol% to 1 vol%, allowing the patient to breathe spontaneously at a normal rate for his age to maintain normoventilation. An inhaled concentration of 1 vol% provides approximately 0.6 - 0.7 MAC on our anesthesia machine, which we expected would not significantly reduce mean arterial pressure.
The final anesthetic goal was to preserve total body volume, which is particularly crucial in MMD patients. They require 1.5 - 2 times the daily fluid intake of a typical child to maintain body fluid volume and prevent dehydration, a regimen necessary for both daily routines and during anesthesia or deep sedation. During the 60-minute MRI, our patient received 90 mL/h of isotonic fluid, 1.5 times greater than the norm for his age, as MRI is a diagnostic procedure with no expected intravascular volume losses. We used isotonic crystalloid Hartmann's solution without added glucose since the boy was permitted to consume sweet fluids up to one hour before anesthesia and was expected to resume oral intake two hours after awakening.
As previously mentioned, surgical procedures in MMD patients do not guarantee definitive results, and in some cases, the outcomes can be unsatisfactory. However, thanks to proficient surgical techniques, effective postoperative care, and diligent parental support, the follow-up findings for our patient were favorable (Figure 2).
Although MRI is a diagnostic procedure, administering anesthesia can be complex. The complexity arises from the uncertainty of postoperative outcomes and the potential impact of anesthesia on compromised cerebral circulation if the surgical results are suboptimal. Thus, it was crucial to meticulously plan the anesthesia approach and select a technique that minimally affects CBF. In this instance, we believe that deep sedation with sevoflurane was successful in providing optimal imaging conditions while satisfactorily managing hemodynamics and respiration—essentially controlling factors that could influence CBF.