1. Background
The coronavirus disease 2019 (COVID-19) pandemic causing acute respiratory distress syndrome has become the world’s most considerable health concern since the beginning of 2020 (1). While the findings were distinct and less common in children initially, it was reportedly to cause a multisystem inflammatory syndrome (MIS-C), which eventually leads to hyperinflammation syndrome over time. Interestingly, MIS-C was detected in children and adolescents who were previously exposed to COVID-19 for 2 - 4 weeks (2). As the cases increase globally, MIS-C has been reportedly characterized by fever, gastrointestinal, coronary artery, and myocardial involvement, and mucocutaneous inflammation. As for the differential diagnosis, Kawasaki syndrome, toxic shock syndrome, bacterial sepsis, and macrophage activation syndrome should be considered (3). However, the specific characteristics of MIS-C include hemodynamic shock syndrome, cardiac failure, and gastrointestinal involvement (4).
According to various publications, cardiovascular findings range from 34% to 82% (3, 4). The majority of studies is composed of cardiac involvement of MIS-C, ventricular dysfunction, coronary artery dilation or aneurysm, and rarely pericarditis and valvulitis (4-6). To date, only a few studies exist regarding the heart function evaluation of patients with MIS-C conducted with conventional tissue Doppler imaging (3, 4, 6-8).
2. Objectives
The present study aimed to compare and assess conventional and tissue Doppler imaging, as well as repetitive cardiac function and laboratory test results, during the follow-up of patients with MIS-C.
3. Methods
3.1. Study Population and Design
This prospective study was conducted between December 2020 and June 2022 at Basaksehir Cam and Sakura City Hospital. Repeated echocardiographic examinations and laboratory tests were conducted on 92 patients. The median age of patients was 7.54 (1.5 - 17.3) years. The Ethics Committee approved this study (date: December 11, 2020; decision no: 09.2020.1304). Before admission, the parents signed a written informed consent form.
3.2. Inclusion Criteria
The study included patients diagnosed with MIS-C who had an echocardiography and laboratory findings at the first admission and at 1-week, 1-month, and 3-month follow-up and who were hospitalized at Basaksehir Cam and Sakura City Hospital. Children between 18 and 208 months were included in the study.
3.3. Exclusion Criteria
Patients with underlying cardiac diseases, acute respiratory distress syndrome, metabolic diseases, chronic liver, renal, and neuromotor diseases, and who had previously received chemotherapy were excluded from the study. Furthermore, patients who could not be scanned with the X5-1 and X7-2 matrix array transducer were removed. Children who were incompatible with echocardiography were removed.
3.4. MIS-C Diagnosis of Confirmed Cases
All suspected hospitalized patients were screened for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus using a nasopharyngeal swab, polymerase chain reaction (PCR), and immunoglobulin G antibodies. The clinical diagnosis of MIS-C was determined based on the criteria established by the World Health Organization and the US Centers for Disease Control and Prevention (4, 9). The MIS-C diagnosis was defined based on clinical signs, multiple system (two or more) organ dysfunction, positive SARS-CoV-2 PCR and/or serological testing, and exclusion of viral and bacterial alternative diagnoses. Patients with an epidemiological relation to a person with COVID-19 were accepted (9).
3.5. Assessment of Laboratory Results
Routine blood tests, including blood cell counts, biochemical profile, and systemic inflammation indicators C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), procalcitonin, ferritin, D-dimer, fibrinogen, and lactate dehydrogenase, interleukin 6 (IL-6), were performed three times: At the first examination, the first week, and the first month. The laboratory results at 3 months post-admission were not reviewed because the patients recovered. Troponin T (ng/L) and proBNP (ng/L) values were assessed upon admission and during hospitalization.
3.6. Cardiovascular Examination
Electrocardiogram and echocardiogram were conducted on each patient admitted to the hospital suspected of having MIS-C. All data were documented, and echocardiogram was performed after the initial admission and at 1 week, 1 month, and 3 months post-procedure.
3.7. Transthoracic Echocardiogram
Transthoracic echocardiogram was carried out with Philips Affiniti 50 (Philips Healthcare, Andover, Netherlands) using an 8S or 5S probe. All patients were examined by a single pediatric cardiologist with extensive experience. All conventional echo modes are employed, including M-mode, 2D, color, pulse and continue wave Doppler (PW-CW), and tissue Doppler. Initial measurements were performed before the inotropic (milrinone) treatment.
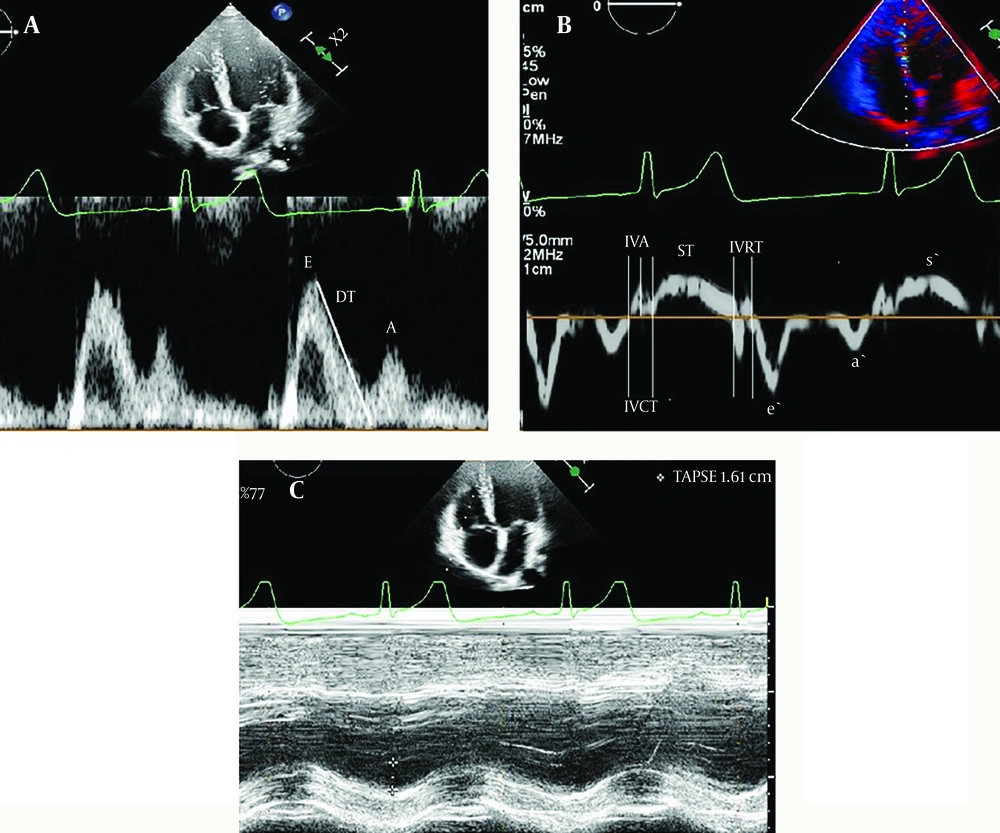
Standard echocardiographic measurements, including fractional shortening (FS), were calculated using the M-mode, ejection fraction (EF), early (E) and late (A) mitral flow peak velocities with spectral Doppler, TAPSE calculated using M-mode, and tissue Doppler with systolic (s) and early diastolic basal septal (e) and lateral mitral annular peak velocities (e′) and systolic (s) and diastolic lateral tricuspid annular peak velocity, peak late diastolic (a), ST, isovolemic acceleration (IVA), isovolumic contraction time (ICT), isovolumic relaxation time (IRT), myocardial performance index (MPI) were performed following the guidelines of the American Society of Echocardiography (10, 11). The mitral lateral E/e' ratio, mitral septal E/e' ratio, and tricuspid lateral E/e' ratio were calculated. Mitral inflow peak E/e' (lateral e' + septal e'/2) was utilized to calculate the average E/e′ ratio. A systolic dysfunction was defined as an FS of < 28% or EF of < 55% by the M-mode.
According to the American Heart Association guidelines for Kawasaki disease, coronary arteries were measured across inner edges, avoiding branch points that may have typical focal dilatation (12). Using the Boston z-score approach, coronary artery anomalies were categorized as follows: < 2, no involvement; ≥ 2 to < 2.5, dilatation; ≥ 2.5 to < 5, tiny aneurysm; ≥ 5 to < 10, medium aneurysm; and large or giant aneurysm.
3.8. Statistical Analysis
Analyses were evaluated using 22 SPSS package programs (Statistical Package for Social Sciences; SPSS Inc., Chicago, IL). In the study, descriptive data are provided as n and % values in categorical data and continuous data as means ± standard deviations (mean ± SD), median values, and Interquartile Range (IQR) [75% - 25%]. Chi-square (Pearson chi-square) analysis was employed to compare categorical variables across groups. The Kolmogorov-Smirnov test assessed the conformity of continuous variables with normal distribution. Patients were divided into two groups: Mitral E/e' ≥ 8 and mitral E/e' < 8 (6, 13). Student’s t-test was used for normally distributed variables, and the Mann-Whitney U-test was used for non-normally distributed variables to compare paired groups. When comparing more than two groups, a repeated analysis of variance (ANOVA) test was used for regularly distributed variables, and Friedman’s ANOVA was used for non-normally distributed variables. For repeated ANOVA, the P-value of the Greenhouse-Geisser test is used when the sphericity assumption is not fulfilled. Bonferroni test was used for two-by-two comparison analysis between multiple groups (pairwise comparisons). When comparing the repeated groups with non-normally distributed parameters, the Wilcoxon signed-rank test was utilized. Pearson’s correlation test was used for normally distributed variables, and the Spearman correlation test for non-normally distributed. A receiver operating characteristic (ROC) curve analysis was performed to determine threshold levels of IL-6, proBNP, CRP, procalcitonin, troponin T, and creatine kinase to predict cardiac diastolic dysfunction. The significance level has been accepted as P < 0.05.
4. Results
4.1. Patient Characteristics
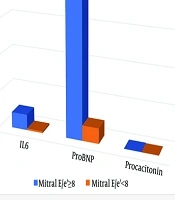
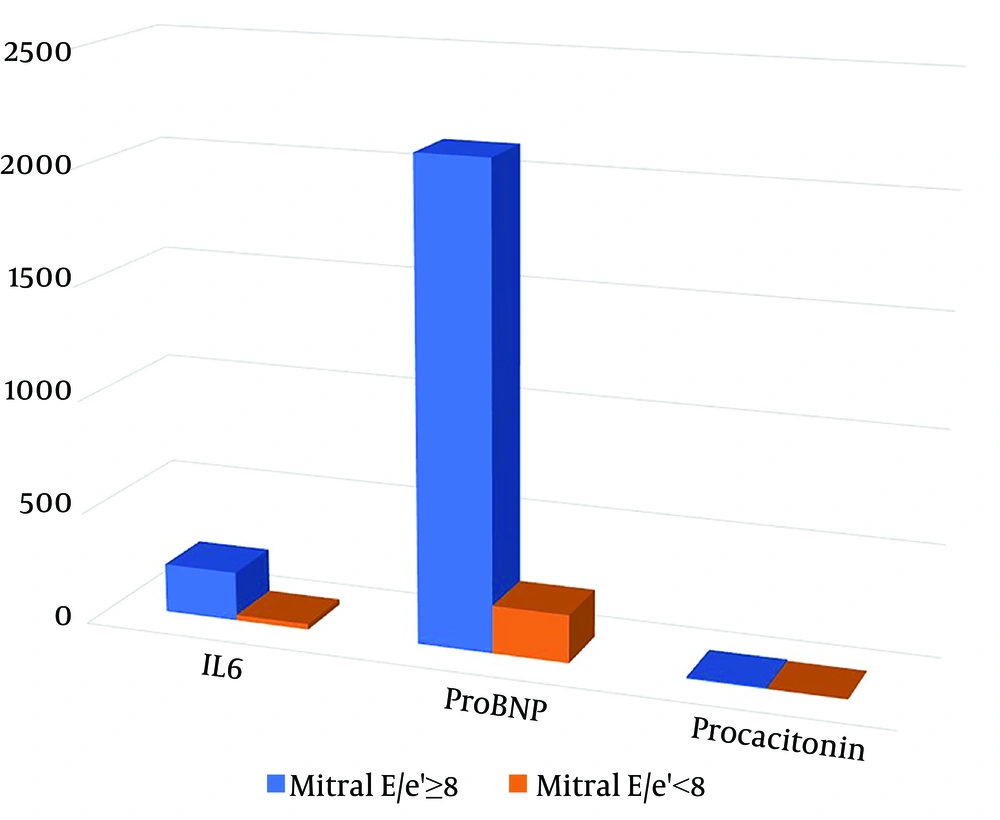
The study included 32 females out of a total of 92 patients with MIS-C [Mitral E/e’(≥ 8):17, Mitral E/e’(< 8):75]. The mean age of the patients was 104.5 ± 52.1 months [Mitral E/e’(≥ 8): 99.7 ± 52.2, Mitral E/e’(< 8): 105.6 ± 52.4]. Six patients were excluded from the study due to insufficient data. The demographic and medical characteristics of the patients are provided in Table 1. All patients with MIS-C had no history of Kawasaki disease. No patients underwent cardiac magnetic resonance imaging. Procalcitonin, ESR, proBNP, ferritin, and IL-6 values were found to be significantly higher in the E/e' ≥ 8 groups compared to the mitral valve E/e' < 8 groups (P < 0.05) (Figure 1). The average hospitalization lasted 11.4 ± 6.2 days. Of the patients, 20% were administered milrinone, 66% with IVIG (2 g/kg), 73% with corticosteroids (2 mg/kg/day in mild and moderate cases; 10 - 30 mg/kg/day for 3 days followed by 2 mg/kg/day in severe cases; and steroid treatment was reduced and discontinued within 4 - 6 weeks), 60% were treated with aspirin (for 2 months), 14% with low-molecular-weight heparin (dosage of 1 mg/kg twice daily for 1 - 2 weeks), 9% with IL1 receptor antagonist (anakinra), and 82% with antibiotics. In 23% of the patients, mitral regurgitation and left ventricular systolic dysfunction were detected.
Two patients demonstrated coronary artery involvement (%2). In one patient, left main coronary artery (LMCA) proximal dilatation (z score: +2.08), LMCA distal, medium aneurysm (z score: +5.5), right coronary artery (RCA) proximal small aneurysm (z score: +2.6), RCA distal, medium aneurysm (z score: +5.7), and a small aneurysm (z score: +4.1) in the left anterior descending (LAD) artery were detected. In another patient, an LMCA distal, medium aneurysm (z score: +5), RCA distal, medium aneurysm (z score: +5.7), and a large aneurysm (z score: +14) in LAD were detected.
| Variabilities | All (n: 92) | Mitral E/e’ (≥ 8), (n = 17) | Mitral E/e’ (< 8), (n = 75) | P-Value |
|---|---|---|---|---|
| Age (mo) | 104.5 ± 52.1 | 99.7 ± 52.2 | 105.6 ± 52.4 | 0.704 |
| Gender | 0.239 | |||
| Female | 32 | 8 | 24 | |
| Male | 60 | 9 | 51 | |
| BMI | 18.0 ± 3.0 | 17.3 ± 2.9 | 18.1 ± 3.6 | 0.508 |
| Hemoglobin | 11.5 ± 1.4 | 11.2 ± 1.4 | 11.6 ± 1.4 | 0.703 |
| WBC | (12.47 - 6.51) 8.65 | (12.47 - 5.940) 10.3 | (13.180 - 6.940) 8.3 | 0.632 |
| Platelet | (266 - 148) 211 | (238 - 98) 166.5 | (267 - 151) 258.5 | 0.034 |
| ALC | (2.17 - 0.92) 1.38 | (2.28 - 0.57) 1.43 | (19.10 - 0.91) 1.51 | 0.442 |
| ANC | (10.2 - 4.2) 6.94 | (8.85 - 3.19) 6.98 | (10.67 - 4.57) 6.39 | 0.810 |
| CRP | 144.5 ± 93.2 | 144.9 ± 74.1 | 144.4 ± 97.5 | 0.486 |
| Procalcitonin | (6.67 - 0.47) 1.76 | (12.2 - 2.41) 6.69 | (5.28 - 0.37) 092 | 0.021 |
| ESR | 42.5 ± 28.2 | 46.5 ± 17.9 | 41.6 ± 30.1 | 0.038 |
| INR | (1.18 - 1.03) 1.11 | (1.28 - 1.08) 1.21 | (5.28 - 0.37) 1.11 | 0.598 |
| D Dimer | (2.99 - 0.64) 1.66 | (3.5 - 1.09) 1.7 | (2.35 - 0.58) 1.21 | 0.283 |
| Creatinin | 0.49 ± 0.21 | 0.48 ± 0.16 | 0.49 ± 0.22 | 0.149 |
| CK | 121.5 ± 243.9 | 53.8 ± 35.6 | 138.4 ± 269.9 | 0.082 |
| Troponin | (16.85 - 3) 3.81 | (52.2 - 6.24) 11.6 | (12.5 - 3) 3 | 0.097 |
| ProBNP | (2204 - 101.7) 260 | (4463 - 394) 2123 | (1281 - 78) 213 | 0.016 |
| Ferritin | (457 - 135) 229 | (655 - 302) 494.5 | (361 - 130) 216 | <0.01 |
| Fibrinogen | 543.2 ± 166.7 | 581.2 ± 163.9 | 535.3 ± 167.4 | 0.351 |
| IL6 | (136.2 - 7.7) 37.95 | (365 - 72) 218 | (105 - 5.87) 24.5 | 0.004 |
| Bilateral lung infiltration | 0.065 | |||
| (+) | 22 | 7 | 15 | |
| (-) | 70 | 10 | 60 | |
| Need for PICU | 0.291 | |||
| (+) | 14 | 4 | 10 | |
| (-) | 78 | 13 | 65 | |
| Stay in hospital (day) | 11.4 ± 6.2 | 11.8 ± 3.7 | 11.3 ± 6.7 | 0.758 |
| Milrinone | 0.07 | |||
| (+) | 18 | 6 | 12 | |
| (-) | 74 | 11 | 63 | |
| IVIG | 0.145 | |||
| (+) | 55 | 14 | 48 | |
| (-) | 37 | 3 | 27 | |
| Corticosteroid | 0.029 | |||
| (+) | 57 | 16 | 51 | |
| (-) | 35 | 11 | 24 | |
| Aspirin | 0.036 | |||
| (+) | 55 | 14 | 41 | |
| (-) | 37 | 3 | 34 | |
| Enoxaparine Na | 0.218 | |||
| (+) | 13 | 4 | 9 | |
| (-) | 79 | 13 | 66 | |
| Antibiotics | 0.658 | |||
| (+) | 84 | 16 | 67 | |
| (-) | 9 | 1 | 8 |
Abbreviations: ICU, Intensive care unit; IVIG, intravenous immunoglobulin; IQR, Interquartile Range.
a Mean, standard deviation, Interquartile Range and median values were used.
4.2. Comparison of Cardiac Outcomes According to Mitral Valve E/e'< 8 and E/e'≥ 8 Groups on the First Admission
The results comparing the cardiac outcomes of mitral valve E/e' < 8 and E/e' ≥ 8 groups are shown in Table 2. When comparing the mitral valve E/e' ≥ 8 group with the E/e'< 8 group, mitral valve e’, a’, s,' and tricuspid valve a’, s,' TAPSE, and septal e,' a’, and LV EF, FS values were found to be significantly lower, whereas mitral valve E, ICT, IRT, MPI, and tricuspid valve ICT, IVA, E/e,' and septal ICT, IVA, IRT, MPI values were found to be significantly higher (P < 0.05) (Figure 2 A, B, C ). No statistical difference was observed between the proximal and distal LCA and RCA and between LCA values and z-score results according to mitral valve E/e' > 8 and mitral valve E/e' < 8 values.
| Variabilities b | All (n: 92) | Mitral E/e’ (≥ 8), (n = 17) | Mitral E/e’ (< 8), (n = 75) | P-Value |
|---|---|---|---|---|
| Mitral valve | ||||
| E | (1.00 - 0.86) 0.97 | (1.00 - 0.93) 1 | (1.00 - 0.86) 0.96 | 0.040 |
| A | 0.66 ± 0.11 | 0.67 ± 0.15 | 0.66 ± 0.11 | 0.094 |
| E/A | 1.45 ± 0.20 | 1.54 ± 0.23 | 1.43 ± 0.19 | 0.445 |
| e’ | (18 - 13.15) 16 | (14 - 9.3) 13 | (18.1 - 14.4) 16.6 | < 0.001 |
| a’ | (10 - 7) 8 | (7 - 5) 7 | (10 - 7) 8.4 | 0.004 |
| s’ | (11 - 7.67) 10 | (9.5 - 6.25) 7.6 | (12 - 8.5) 10 | 0.003 |
| ICT | (53 - 40) 42 | (72.5 - 49) 53 | (50 - 37) 42 | 0.041 |
| IRT | (61 - 40) 48 | (62 - 53) 61 | (61 - 40) 45 | 0.017 |
| E/e’ | 6.97 ± 1.56 | 10.14 ± 2.12 | 6.36 ± 1.00 | 0.003 |
| MPI | (0.44 - 0.32) 0.36 | (0.56 - 0.44) 0.45 | (0.41 - 0.32) 0.35 | 0.001 |
| Tricuspid valve | ||||
| E | 0.75 ± 0.15 | 0.72 ± 0.10 | 0.76 ± 0.16 | 0.101 |
| A | (0.62 - 0.45) 0.54 | (0.59 - 0.46) 0.53 | (0.65 - 0.43) 0.54 | 0.922 |
| E/A | (1.51 - 1.23) 1.37 | (1.50 - 1.21) 1.33 | (1.54 - 1.23) 1.37 | 0.499 |
| e’ | (19.00 - 15.45) 17 | (19 - 12) 15 | (19 - 16) 17 | 0.210 |
| a’ | (13.55 - 8.95) 11 | (11 - 6.1) 8.5 | (13.8 - 9.1) 11 | 0.041 |
| s’ | (15.00 - 12.22) 14 | (13.3 - 11) 12 | (15 - 13) 14 | 0.004 |
| ICT | 50.8 ± 12.4 | 54.07 ± 19.5 | 50.2 ± 10.7 | 0.006 |
| IVA | 28.4 ± 7.4 | 32.8 ± 12.04 | 27.5 ± 5.9 | < 0.001 |
| E/e’ | (5.16 - 3.46) 4.1 | (5.58 - 3.85) 5.4 | (5.02 - 3.31) 4.75 | 0.011 |
| MPI | (0.44 - 0.34) 0.38 | (0.42 - 0.33) 0.37 | (0.44 - 0.34) 0.38 | 0.972 |
| TAPSE | 22.6 ± 5.4 | 17.7 ± 4 | 23.6 ± 5.2 | < 0.001 |
| Septum | ||||
| e’ | (14.25 - 11.00) 13 | (9.5 - 7.35) 9 | (15 - 11.35) 13 | < 0.001 |
| a’ | (9.00 - 6.5) 8 | (7.5 - 5) 6 | (9.25 - 7.1) 8 | 0.008 |
| s’ | 8.29 ± 1.5 | 6.69 ± 1.0 | 8.6 ± 1.4 | 0.118 |
| ICT | (53 - 37) 45 | (63.5 - 42) 55 | (51.5 - 37) 45 | 0.007 |
| IVA | (29 - 18) 24 | (32 - 22.5) 32 | (29 - 18) 24 | 0.037 |
| IRT | (53.5 - 42) 48 | (62 - 46.5) 58 | (51.5 - 42) 48 | 0.008 |
| MPI | (0.40 - 0.30) 0.34 | (0.48 - 0.32) 0.40 | (0.37 - 0.30) 0.34 | 0.022 |
| Left ventricular function | ||||
| EF (%) | (72 - 59) 67.6 | (64 - 49.4) 61.5 | (73 - 62) 68.5 | 0.026 |
| FS (%) | (41 - 30) 37 | (34.5 - 24.8) 32.5 | (41 - 31.5) 37 | 0.048 |
Abbreviations: ICT, isovolumic contraction time; IVA, isovolumic acceleration; IRT, isovolumic relaxation time; IQR, Interquartile Range; MPI, myocard performance index; EF, ejection fraction; FS, fraction of short.
a Mean, standard deviation, Interquartile Range and median values were used.
b A: Mitral-tricuspid inflow peak A wave velocity (active atrial contraction), a: Atrial contraction velocity with tissue Doppler imaging, E: Mitral-tricuspid inflow peak velocity of early filling, e: Early diastolic velocity with tissue Doppler imaging, s: Systolic flow velocity with tissue Doppler imaging.
4.3. Repeated Tissue Doppler Imaging Findings
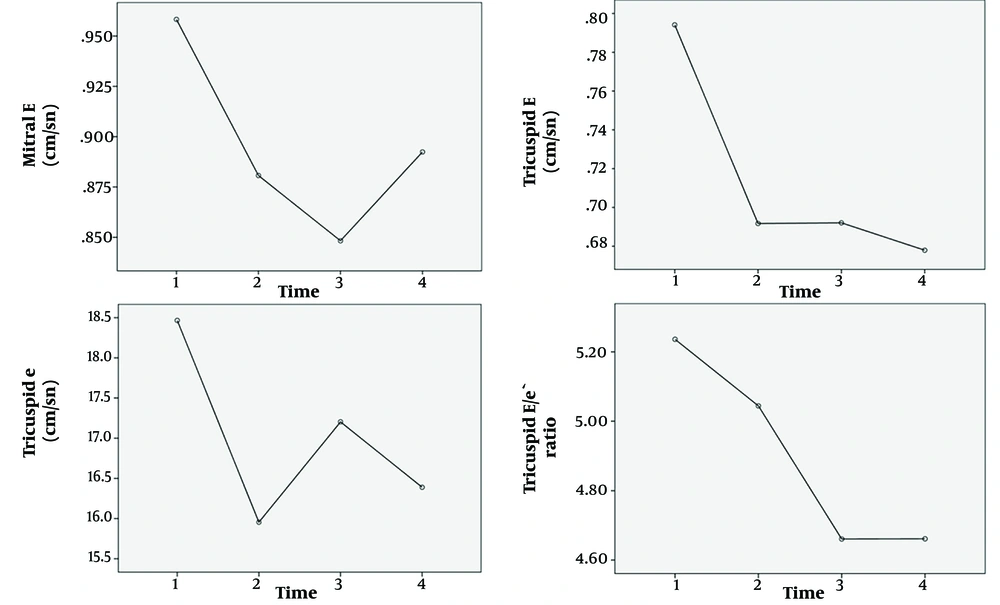
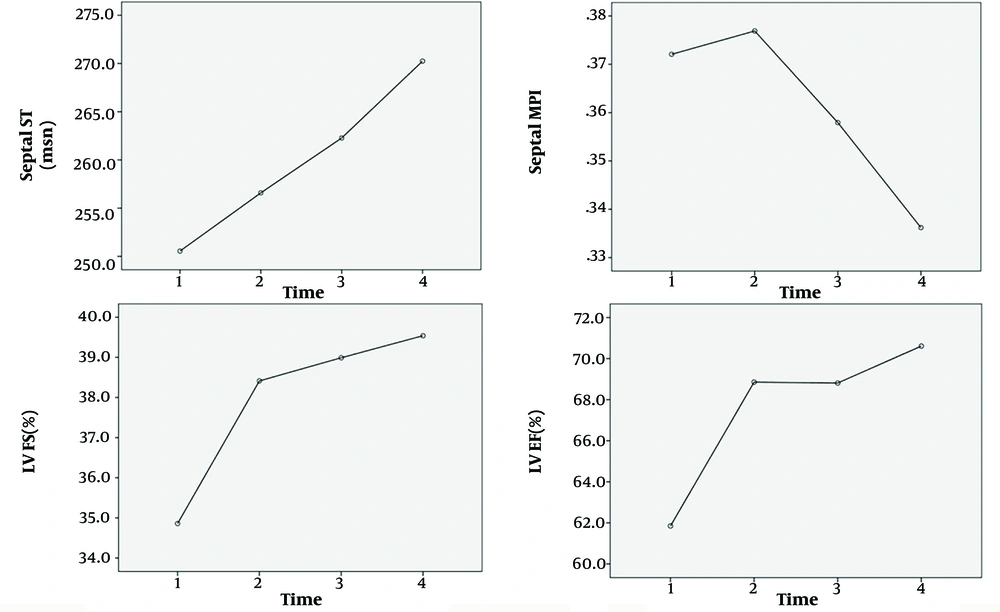
Outcomes of repetitive tissue Doppler tests examining cardiac diastolic functioning are shown in Table 3. Statistically significant variations were observed between serial measurements of mitral valve E, tricuspid valve E, e’, E/e', septal ST, MPI, and left ventricular EF and FS across time (Figures 3 and 4; P < 0.05). Among these results, septal ST, left ventricular EF, and FS values were established, which were low during the initial examination, and the mitral valve E, tricuspid valve E, e’, E/e,' and septum MPI values, which were elevated upon initial admission, improved with serial measurements.
| Mitral Valve | Initial | 1 Week Later | P0 | At 1. mo | At 3. mo | P1 | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E (cm/sn) | 95 ± 16 | 88 ± 10 | 0.209 | 84 ± 12 | 89 ± 12 | 0.451 | 1.000 | 1.000 | 0.006 | 0.381 | 0.010 |
| A (cm/sn) | 67 ± 11 | 63 ± 10 | 0.656 | 61 ± 13 | 60 ± 8 | 1.000 | 1.000 | 1.000 | 0.318 | 0.106 | 0.079 |
| E/A ratio | 1.40 ± 1.90 | 1.36 ± 0.2 | 1.000 | 1.40 ± 0.22 | 1.49 ± 0.25 | 0.639 | 1.000 | 1.000 | 1.000 | 0.872 | 0.156 |
| e (cm/sn) | 15.4 ± 3.9 | 15.6 ± 3.5 | 1.000 | 14.7 ± 2.3 | 15.8 ± 3.8 | 0.539 | 0.687 | 1.000 | 1.000 | 1.000 | 0.346 |
| ST (sn) | 253.8 ± 39.5 | 253.7 ± 37.9 | 1.000 | 259.1 ± 38.3 | 264.3 ± 29.6 | 1.000 | 1.000 | 1.000 | 1.000 | 0.992 | 0.555 |
| M-L E/e' r | 6.37 ± 1.7 | 6.05 ± 1.6 | 0.250 | 5.7 ± 1.0 | 5.9 ± 1.5 | 0.218 | 0.770 | 1.000 | 1.000 | 1.000 | 0.711 |
| Mean E/e' r | 6.56 ± 1.60 | 5.95 ± 1.92 | 0.449 | 5.93 ± 1.18 | 8.43 ± 13.84 | 1.000 | 1.000 | 1.000 | 0.264 | 0.399 | 0.078 |
| MPI | 0.355 ± 0.08 | 0.378 ± 0.07 | 1.000 | 0.352 ± 0.06 | 0.352 ± 0.06 | 1.000 | 1.000 | 0.980 | 1.000 | 1.000 | 0.457 |
| Tricuspid valve | |||||||||||
| E (cm/sn) | 78 ± 16 | 69 ± 13 | 0.077 | 69 ± 11 | 67 ± 11 | 1.000 | 1.000 | 1.000 | 0.039 | 0.006 | 0.004 |
| A (cm/sn) | 83 ± 13.7 | 51 ± 10 | 1.000 | 50 ± 9 | 47 ± 9 | 0.933 | 1.000 | 0.813 | 1.000 | 1.000 | 0.204 |
| E/A ratio | 1.36 ± 0.19 | 1.37 ± 0.23 | 1.000 | 1.38 ± 0.25 | 1.43 ± 0.17 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.682 |
| E (cm/sn) | 18.4 ± 5.14 | 15.9 ± 3.48 | 0.083 | 17.2 ± 3.16 | 16.3 ± 2.49 | 1.000 | 0.819 | 1.000 | 1.000 | 0.330 | 0.031 |
| ST (sn) | 243.5 ± 39.6 | 250.1 ± 36.6 | 1.000 | 253.4 ± 31.6 | 257.3 ± 28.1 | 1.000 | 1.000 | 1.000 | 1.000 | 0.305 | 0.276 |
| Mean E/e' r | 5.28 ± 1.10 | 5.06 ± 1.20 | 1.000 | 4.63 ± 0.87 | 4.66 ± 0.95 | 1.000 | 0.486 | 0.593 | 0.036 | 0.026 | 0.010 |
| MPI | 0.397 ± 0.07 | 0.378 ± 0.08 | 1.000 | 0.364 ± 0.06 | 0.367 ± 0.05 | 1.000 | 1.000 | 1.000 | 0.525 | 0.331 | 0.176 |
| TAPSE (mm) | 21.1 ± 4.7 | 21.2 ± 3.2 | 1.000 | 22.3 ± 3.5 | 22.6 ± 2.6 | 1.000 | 0.912 | 0.389 | 1.000 | 0.978 | 0.189 |
| Septal | |||||||||||
| e(cm/sn) | 12.7 ± 2.9 | 11.9 ± 2.1 | 0.746 | 12.4 ± 2.4 | 12.9 ± 1.8 | 1.000 | 1.000 | 0.210 | 1.000 | 1.000 | 0.185 |
| ST (sn) | 250.5 ± 38.6 | 256.5 ± 27.7 | 1.000 | 262.2 ± 30.5 | 270.2 ± 37.2 | 0.938 | 1.000 | 0.334 | 0.330 | 0.067 | 0.031 |
| M-S E/e' r | 7.8 ± 2.0 | 7.5 ± 1.7 | 1.000 | 6.9 ± 1.4 | 6.9 ± 1.2 | 0.165 | 0.146 | 0.832 | 1.000 | 1.000 | 0.082 |
| MPI | 0.37 ± 0.08 | 0.37 ± 0.04 | 1.000 | 0.35 ± 0.07 | 0.33 ± 0.05 | 0.487 | 0.795 | 0.003 | 1.000 | 0.276 | 0.038 |
| LV function | |||||||||||
| FS (%) | 34.8 ± 7.8 | 38.4 ± 5.9 | 0.002 | 38.9 ± 3.7 | 39.5 ± 3.9 | 1.000 | 1.000 | 1.000 | 0.003 | 0.001 | <0.01 |
| EF (%) | 61.8 ± 12.2 | 68.8 ± 8.3 | 0.004 | 68.8 ± 5.5 | 70.6 ± 4.6 | 0.900 | 1.000 | 1.000 | 0.021 | 0.006 | <0.01 |
a A: Mitral-tricuspid inflow peak A wave velocity(active atrial contraction), E: Mitral-tricuspid inflow peak velocity of early filling, e: Early diastolic velocity with tissue Doppler imaging, M-L E/e' r: Mitral lateral E/e' ratio, M-S E/e' r: Mitral septal E/e' ratio, ST: Systolic time, MPI: Myocard performance index, EF: Ejection fraction, FS: Fraction of short, P0 – P-value between the values initial and 1 week later; P1-P-values between the values of patients at the first month and values of patients at the third month; P2-P-values between the values of patients of 1 week later and values of patients at the first month; P3-P-values between the values of patients of 1 week later and values of patients at the third month; P4 - P-values between the values of initial and values of patients at the first month, P5 - P-values between the the values of initial and values of patients at the third month; P6 - P-value for assessment of all values, TAPSE: Tricuspid annular plane systolic excursion, T-L E/e' r: Tricuspid lateral E/e' ratio
4.4. Results of the Correlation Between mitral E/e’ and Some Biochemical Parameters on the First Admission
Outcomes of the correlation between mitral E/e’ and some biochemical parameters are shown Table 4. Statistically significant positive correlation as observed between the initial mitral E/e’ and IL-6, ferritin, proBNP, troponin T, procalcitonin, CRP, ESR, and fibrinogen values.
| Variables | P | r |
|---|---|---|
| Initial Mitral E/e’ | ||
| Interleukin-6 | 0.010 | 0.296 |
| Ferritin | < 0.001 | 0.428 |
| ProBNP | 0.001 | 0.353 |
| Troponin T | < 0.001 | 0.392 |
| Procalcitonin | 0.024 | 0.252 |
| CRP | 0.025 | 0.248 |
| ESR | 0.015 | 0.269 |
| Fibrinogen | 0.026 | 0.253 |
| Initial Mitral E/e’ | P | r |
| Interleukin-6 | 0.010 | 0.296 |
| Ferritin | < 0.001 | 0.428 |
| ProBNP | 0.001 | 0.353 |
| Troponin T | < 0.001 | 0.392 |
| Procalcitonin | 0.024 | 0.252 |
| CRP | 0.025 | 0.248 |
| ESR | 0.015 | 0.269 |
| Fibrinogen | 0.026 | 0.253 |
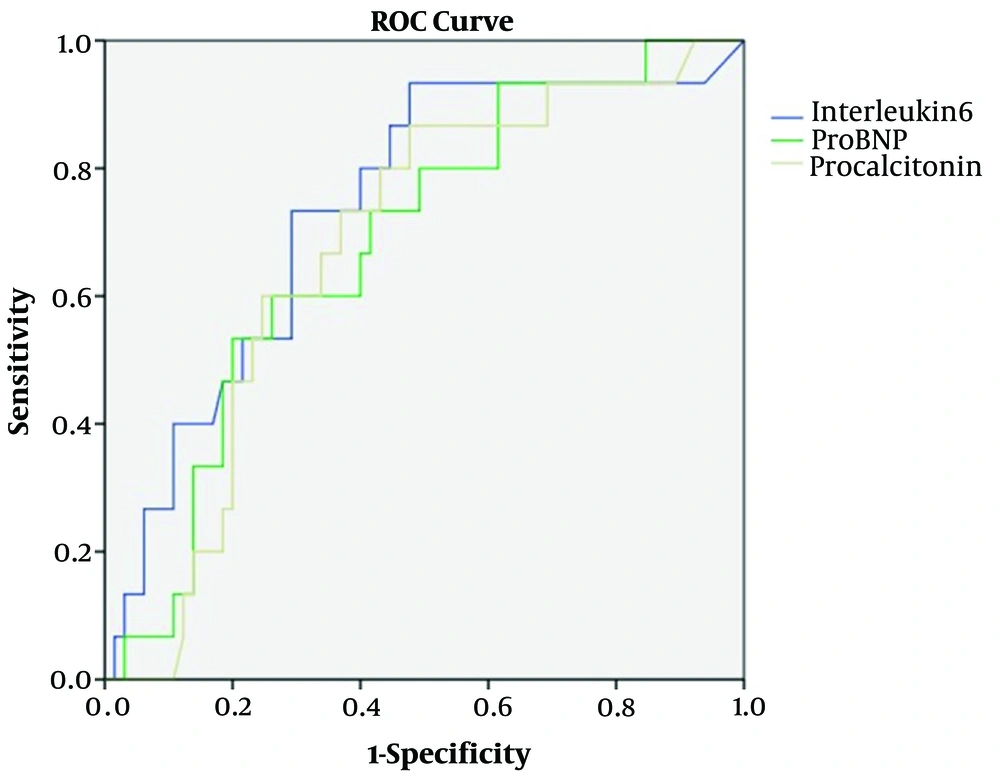
4.5. Results of ROC Analysis According to Mitral E/e' and Some Biochemical Parameters on the First Admission
In the ROC curve analysis, IL-6 of > 70.3, proBNP of > 278.5, and procalcitonin of > 2.4 were identified as effective cut-off points in the mitral E/e' (> 8) for patients with MIS-C (area under the curve (AUC) = 0. 743, 95% confidence interval (CI) = 0.599 - 0.887, P = 0.005, for IL-6; AUC = 0. 680, 95% CI = 0.535 - 0.825, P = 0.037, for proBNP; AUC = 0.672, 95% CI = 0.528 - 0.816, P = 0.046, for procalcitonin). The IL-6, proBNP, and procalcitonin values of >70.3, >278.5, and >2.4 yielded sensitivity and specificity values of 72% and 74%, 72% and 60%, and 72% and 60%, respectively (Figure 5).
5. Discussion
The most noteworthy results of our study are as follows:
(1) The mitral valve E, tricuspid valve E, e, E/e', septal ST, MPI, and M-mode values in patients with MIS-C measured serially by tissue Doppler imaging, as well as left ventricular EF and FS values showed significant differences and improvements in final measurements.
(2) Mitral valve E/e’> 8 appears to be significantly associated with IL-6, proBNP, and procalcitonin values in patients with MIS-C.
(3) IL-6, proBNP, and procalcitonin values were determined as independent high-risk indicators for left ventricular diastolic dysfunction.
The incidence of MIS-C in children infected with SARS-CoV-2 was reported as 0.03 - 0.6% in patients with MIS-C requiring intensive care support. To our knowledge, 70 MIS-C deaths have been reported in the United States alone (2, 9, 14). MIS-C is a post-viral myocarditis usually involving shock, left ventricular systolic and diastolic dysfunction, AV valve regurgitations, pericardial effusion, or coronary artery aneurysms (6, 7, 14-16). Awareness of diastolic dysfunction at the initial presentation may indicate a more serious disease course. Precautions can be taken accordingly. Cardiac involvement in patients with MIS-C has been reported in several previous studies (3, 6-8). In these studies, the prevalence of LV dysfunction ranges between 11% and 50% with the M-mode (6-8, 16). Theocharis et al. reported valve regurgitation in 75% of patients, whereas Yakut et al. (16) found valve regurgitation in 22% of patients (8). Of the patients, 14% of patients in our study had LV systolic dysfunction, and 23% of patients had mitral valve regurgitation. As a result of serial measurements, we detected that our study’s FS and EF values have improved with time. MIS-C refers to the inflammatory response following SARS-CoV2 infection. The hyperinflammatory response in children with acute untreated MIS-C is characterized by elevated levels of IL-6 and other cytokines. The hyperinflammatory response is also associated with endothelial dysfunction and microangiopathy. Inflammatory markers (proBNP, procalcitonin) associated with vascular inflammation and myocardial damage are elevated in patients with MIS-C (17).
Conventional tissue Doppler imaging has been utilized in previous studies to evaluate the diastolic function of the ventricles (6, 8). Only a few tissue Doppler imaging studies have provided information on diastolic dysfunction in patients with MIS-C (6, 8). This study has the highest number of patients who underwent tissue Doppler imaging. Matsubara et al. (7) compared 20 patients with MIS-C to a control group and found no statistically significant differences in the E/A ratio, septal mitral e, or lateral e/septal e ratio values, whereas statistically significant differences among the lateral mitral e, averaged E/e ratio, and TAPSE value were found. Kavurt et al. (6) reported that the left ventricular function parameters as mitral lateral-septal s’ velocities were found to be significantly lower in the 3D LVEF ≥ 55% group compared to the control group. In our study, a significant difference was observed in mitral lateral e', a', s,' tricuspid lateral a', s,' ICT, IVA, E/e', TAPSE, and septal e,' a', ICT, IVA, IRT, and MPI measurements between the E/e ratio > 8 and E/e ratio < 8 groups. These values at the initial presentation support the diastolic dysfunction in both the right and left ventricles. In both groups, inflammatory markers were higher in the group with diastolic dysfunction. Furthermore, a significant correlation was observed between the initial mitral E/e' and important inflammatory markers. Accordingly, inflammation seems to contribute to diastolic dysfunction.
Studies related to long-term diastolic dysfunction are rarely conducted. Sanil et al. (15) reported that impaired left ventricular global longitudinal strain (LVGLS) and left ventricular apical four-chamber peak longitudinal strain (LVA4LS) values at the first admission were higher independent risk factors for an adverse acute clinical course and persistent subclinical left ventricular dysfunction at the 10-week follow-up. In our study, significant differences were observed between serial tricuspid E, e', and E/e ratio measurements. When comparing tissue Doppler studies and this study, diastolic dysfunction characteristics of patients with MIS-C were found to have improved over time. In addition to these results, improvement in the right ventricular tricuspid E wave, e', E/e' ratio, and septal MPI suggests that right ventricular parameters can be used with left ventricular parameters in patients with MIS-C. Our study may demonstrate that it is relevant to the disease process, especially because systolic function was generally normal in their cohort. The study seems to infer that changes in diastolic parameters signify diastolic dysfunction with a pseudonormalization pattern. Patients with MIS-C appear to have diastolic dysfunction for at least 3 months. Patients with MIS-C were recommended to have serial echocardiographic follow-up to monitor improvement in diastolic dysfunction over time.
In their study, Kavurt et al. (6) compared patients with MIS-C to those with 3D LV EF < 55% vs. >55%. Troponin I, NT-proBNP, procalcitonin, and ferritin levels were significantly elevated in the group with low EF. Kelly et al. (18) demonstrated that patients with poor LVEF had elevated troponin I, Pro BNP, ferritin, and D-dimer levels, which reverted to normal during the recovery stages. McAree et al. (14) reported that higher peak CRP-values were associated with lower LVGLS and circumferential strain during follow-up. They indicated that a peak CRP value of < 18 mg/dL during an acute illness may indicate a lower risk of myocardial dysfunction during follow-up. Yakut et al. (16) reported that troponin T, proBNP, and IL-6 levels could predict the risk of cardiac involvement in patients with MIS-C. They showed that elevated troponin T (> 11.65 ng/L), proBNP (> 849.5 pg/mL), and IL-6 (> 39.8 pg/mL) thresholds may be a predictor of left ventricular systolic dysfunction on echocardiographic examinations. In this study, we show that IL-6 of > 70.3, proBNP of > 278.5, and procalcitonin of > 2.4 may be a predictor for left ventricular diastolic dysfunction in echocardiographic examinations.
To reduce false-positive diagnoses of diastolic dysfunction, it is recommended to look for the presence of several abnormal findings and cut-off values with high specificity for myocardial disease. The proposed variables are annular e' velocity (septal e,' lateral e') and E/e' ratio. Apart from this, mitral E and E/A ratios are less frequently used parameters for diastolic dysfunction in the literature (19). When Table 2 is analyzed, statistically significant differences between septal e' and mitral e' values based on mitral E/e'> 8 and mitral E/e'<8 support the diastolic dysfunction. Dragulescu et al. reported that the diagnostic criteria are insufficient to evaluate diastolic dysfunction in pediatric patients. They reported that normal pediatric reference values have a wide range, allowing the diagnosis of diastolic dysfunction in only a small proportion of patients (20). In the 2016 JASE guideline (Nagueh et al.), mitral E/e' <10 (previously 8 - 9) was reported as grade I, 10 - 14 as grade II, and >14 as grade III (19). However, in our study, we took the smallest possible cut-off value of 8 for mitral E/e'. Therefore, the minimum values that would cause diastolic dysfunction were considered. Although the number of patients with RCM was very small according to Sasaki et al., E/e' values (lateral: 6.08 ± 3.4; 3.8 ± 1.77, septal:10.26 ± 7.7; 5.2 ± 2.2) were found to be statistically significantly higher compared to the control group. The mean e' value was not used in this study. The small number of patients in this study may have caused variability in diastolic dysfunction (21).
IL-6, proBNP, and procalcitonin levels in MIS-C patients are always analyzed at the first presentation. The IL-6, proBNP and procalcitonin levels > 70.3, > 278.5 and > 2.4, respectively, suggest that these patients may be at high risk for left ventricular diastolic dysfunction. According to these results, a proactive approach can be taken in the management of patients.
The study’s primary shortcomings, potential for selection biases, and limitations are its single-center design, relatively small sample size, and lack of comparison to a control group. In addition, infant patients were not included in the study since they could not fully participate and might cry during echocardiography. The study was unequally comprised of males and females, which can affect the data. Another limitation is that milrinone treatment affects cardiac functions, except at the first admission. Moreover, this study has no intra- and inter-observer reproducibility. However, at least three images were captured during echocardiography. Measurements were taken by selecting the most appropriate and best image for TDI, which is the most experienced pediatric cardiologist. Although the mitral valve E/e'> 8 value used for adult patients is a disadvantage for this study, it emphasizes the importance of obtaining significant results in children. The small number of patients in the group with mitral valve E/e'> 8 may be a potential bias. To overcome these limitations, studies with a larger number of patients should be conducted with a control group. Furthermore, intra- and inter-observer reproducibility are recommended for future studies.
In conclusion, serially measured tissue Doppler imaging diastolic dysfunction parameters seem to be improved until the 3-month follow-up in patients with MIS-C. Mitral valve E/e’> 8 appears to be significantly associated with IL-6, proBNP, and procalcitonin in patients with MIS-C. IL-6, proBNP, and procalcitonin values were determined as independent high-risk indicators for left ventricular diastolic dysfunction. It is important to follow up on asymptomatic patients with MIS-C in terms of cardiac sequelae due to ongoing diastolic dysfunction.